‘Controlling the Mind and Slicing the Body with Light’ will explore optogenetics - an intriguing technique that has been used to track brain activity, cause cancer cells to glow, and even control a rat’s brain. We asked the three speakers, based at University College London, to give us a taster.
1
Mark Lythgoe, Professor of Biomedical Imaging and Co-director of the festival
Focus: What can people expect from your event?
ML: The talk’s split into three parts – the optogenetics and ‘light sheet’ work I’m going to be covering, then Amit Jathoul will talk about imaging cancer cells, and a final part by Clare Elwell, who has developed a technique that measures the blood supply in the brains of babies. We’re also going to do a live experiment, where we will use a technique that illuminates a whole fruit fly embryo to create a three-dimensional image. We’ll scan it during the lecture and at the end we’ll be showing the pictures.
What is optogenetics?
I am very excited about this technology. You can penetrate the body with light and then get information out of it, or actually activate the body. We make the brain light-sensitive, so that then you can switch the brain on or off. Quite simply, we’re able to control the brain using light. There is definitely a wow factor about it.
Your talk title mentions ‘slicing the body with light’. What do you mean by this?
If you take a fruit fly or zebrafish embryo, which is nearly transparent, you can shine light all the way through it. It’s done with a sheet of light. We start with one sheet, then the next slice, then the next slice, so we can slice completely through an embryo without destroying it. We can genetically modify the embryo to give out light, so the light sheet penetrates the body and then light comes out, and we can use this light to develop a three-dimensional picture of the cells and the cellular structure.
How has optogenetics changed brain research?
Previously, you would put an electrode into the brain and it would activate all the cells. With optogenetics, you can specifically activate just one cell type, and that’s the first time we’ve been able to do that. So you’re able to separate out the individual mechanisms involved in brain function and consciousness.
How could it be used in the future?
In the last couple of years, we’ve had deep brain stimulation, which is a treatment where they electrically stimulate the brain using electrodes. In the future, you can imagine more selectively targeted optical stimulation. We’ve also been trying an experiment here to control heart function, so instead of electrical pacemakers, you would have optical pacemakers.
It depends how you define the mind, but certainly you can control the brain. One of the experiments that we’re showing is from Karl Deisseroth. He’s been able to place a little fibre-optic into the brain of a mouse, and then when you switch the light on, you can actually make the animal go in particular directions – for example, it will run round in circles.
Will optogenetics ever be used in humans?
If you had asked me this question about 10 years ago, I would have been very, very skeptical, but the advances in molecular genetics – the ability to modify a cell and then put it back into a human – has come on leaps and bounds. I think the technology is now there, although there are safety issues and legislative issues to overcome. So theoretically there is no reason why you couldn’t optically activate people.
2
Amit Jathoul, Research Associate
Focus: What will your part of the show be about?
AJ: I’m going to be talking about how we take genes from nature and alter them, and how powerful that is. For example, we look at fluorescent corals – they have genes that produce really vibrant, fluorescent proteins. We take these genes out, engineer them so they’re compatible with animals, and use them to light up biochemical pathways in a mouse. We can use this to investigate how well a therapeutic agent will kill a cancer.
How do you do that?
The most common thing I do is to re-engineer genes from fireflies. We engineer a cancer to contain those genes, so that it glows very brightly as it grows inside a mouse. All we have to do is put the mouse in a black box with a decent camera, and you can see it. Then, when we give the mouse an anti-cancer therapy, we can evaluate how effective it is by the extent to which it turns the light off.
3
Clare Elwell, Professor of Medical Physics
Focus: Can you tell us about your part of the show?
CE: I’m a physicist, and I develop systems to image the brain. I’m going to be talking about how this technology works and then show some examples of where we have been using it, including a project that we did recently where we took our imaging equipment out to Africa.
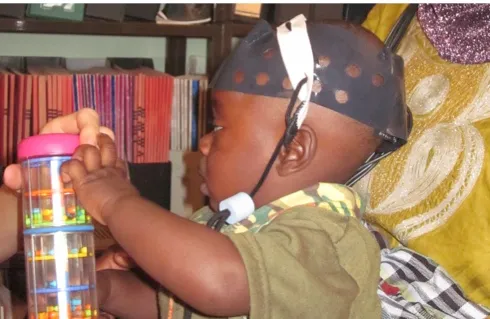
What did this project in Africa involve?
We looked at early markers of autism in really young infants. The system basically maps the distribution of oxygen in the baby’s brain. What we do is put a cap on the baby’s head, and then they sit down on their mum’s lap and look at the screen.On the screen there’s images displayed of tractors and cars and planes, and in between those we have images of an actress doing peek-a-boo. We’ve shown that babies who are at risk of autism respond differently to the actress images, as young as four months of age. And if you can provide an early marker, then you can start intervening earlier.
Follow Science Focus onTwitter,Facebook, Instagramand Flipboard