When we think of a robot, what usually comes to mind is some kind of synthetic servant – a metal-clad machine controlled by electronics. While it might do chores for us and perhaps even talk to us in ways that seem intelligent, we wouldn’t regard it as alive.
But what if, instead of building robots out of hard, lifeless materials, we built them out of the soft materials that nature relies on? What if we built them out of cells?
This is exactly the approach that researchers in Prof Josh Bongard’s lab at the University of Vermont in the US are taking.
For the last four years, they have been designing and creating ‘xenobots’: miniature machines made from living frog cells.
Bongard explains the team’s approach: “[If you] make a robot out of metal and plastic … the pieces themselves have no intelligence.
"We’re approaching robotics in a completely different way. We’re building from components that are themselves fantastically intelligent machines.”
Nature has been inspiring robotics for decades. It’s led to actuators based on real muscles that allow robots to move more easily. Elsewhere, pads that mimic geckos’ feet let robots climb vertical glass. Xenobots, by contrast, are made from nature’s own building blocks.
According to Dr Victoria Webster-Wood, an expert in biologically inspired robots at Carnegie Mellon University, this type of approach “enables us to directly harness living materials’ natural adaptability.”
What’s fascinating about Bongard’s xenobots is that they can be made from normal cells taken from frog embryos – no genetic tweaks required.
Although scientists already knew these cells could move on their own, in this case they’re being used as materials to generate predictable, robot-like behaviours, such as herding particles around a Petri dish, cooperating like sheepdogs and even birthing balls of other cells that might be regarded as xenobot babies.
Artificial selection
While it’s not clear what it is in the xenobots’ internal workings, or rather those of the frog cells, that make them behave in this way, their capabilities do make them potentially useful for all manner of tasks.
Cleaning-up microplastics, for example, or, as the researchers outlined in their first paper on the xenobots, published in 2020, crawling to the site of diseased tissues in humans to help restore them to health.
So if you’re going to make a xenobot, where do you start? Well, the Vermont team starts in a virtual Petri dish, on a computer, where an artificial intelligence (AI) program ‘evolves’ bunches of frog cells, based on their shape, to perform whatever task it is the scientists are interested in.

“It creates a population of virtual xenobots, deletes the ones that do a poor job and makes randomly modified copies of the survivors,” explains Bongard.
The scientists tell the AI how many rounds of this artificial selection process to complete and in just a few seconds, they have their design.
As an example, that design might be a ball of cells with a hole in the middle, like a pouch, which works well for transporting objects.
It’s the AI-based design process that’s the “real masterpiece” of the team’s approach, according to Dr Falk Tauber, a bio-inspired technology expert based at the University of Freiberg in Germany.
Without the virtual Petri dish, he notes, testing hundreds of different cell configurations could take weeks or even months using real cells.
“This not only represents an immense time advantage, but also provides the opportunity to implement only the most promising approaches that have proven successful in [the computer],” he says.
He suggests the AI approach could be useful in other scenarios too – like the rapid design of personalised organ transplants that precisely fit a patient’s anatomy.
Read more:
- Living robots that are capable of self-replicating created in US lab
- Meet the autonomous Moon robots about to change space travel forever
Then comes the time-consuming bit, as the virtual designs have to be transferred to the real-life cells. It’s a process that takes the team’s sole xenobot sculptor, biologist Dr Doug Blackiston, based at Tufts University, Massachusetts, hours for each millimetre-scale xenobot.
Using microsurgery instruments, Blackiston painstakingly carves the shape designed by the AI into tissue harvested from frog embryos.
“For me, it’s a lot like drawing or working on art,” he says, adding that he enjoys seeing the shapes come together.
However, he admits that for the xenobots to find real-world applications, they’ll need to speed up the process to create more than the current 30 to 40 xenobots a week. That advance could come from 3D printing, which can use cells and tissues as printing ‘inks’.
Pushing to progress
The xenobots then spend a little over a week crawling or swimming around a dish before disintegrating (as they don’t eat, their lifespan is limited).
In their original experiments, the researchers made ‘walking’ xenobots from combinations of heart and skin cells; the piston-like action of heart cells translated into movement.
Now, though, they use skin cells, taking advantage of beating hair-like structures called cilia, which protrude from the outer surface of the balls of cells, allowing them to ‘swim’.
After initially seeing their movements, the researchers thought the xenobots might be capable of pushing things around, though they wondered if the xenobots would be strong enough.
“I started with very light dye particles, littered across the bottom of the Petri dish like a fine layer of ash or snow,” says Blackiston. “I happened to get lucky on the first try and it worked.” The xenobots could also push tiny glass beads around.
Read more:
- Miniature T-1000-style robot can shape shift between liquid and solid states
- This shape-shifting technology allows ground robots to morph into flying drones
After the researchers progressed to making the swimming xenobots, they started giving swarms of them more interesting things to move around, like cells – the same cells of which the miniature robots themselves are composed.
That was when something intriguing began to happen: the swarm began pushing the cells into little piles. Frog cells are sticky so the piles tended to stay together and then, a few days later, hairs started to appear on their surface – cilia, just like on the surface of the xenobots.
“And then,” says Bongard, “Doug [Blackiston] noticed that a couple of them started to move.”
At this point, it was clear that the xenobots were making more of themselves. It wasn’t a traditional ‘have sex, make a baby’ type of scenario, but it was a form of replication that had never been seen in nature.
According to Blackiston, he knew the experiment would work if he could get the conditions right. But that didn’t make it any less exciting when he first showed Bongard and the team one of the ‘children’ moving around on a Zoom call.
“It was silent on the call – the biologists, the computer scientists,” Bongard remembers. “You know, self-replication is kind of a dream and a hope [for] machines in general and to see it… it completely blew my mind.”
When the researchers realised their xenobots could self-replicate, they told their AI to evolve versions that could do it better.
The AI set to work, designing a shape that looked pretty familiar: Pac-Man, or as Bongard puts it “a shovel, basically”, which makes sense when you’re making your babies by pushing bits of them into piles – you can see an image of this below.
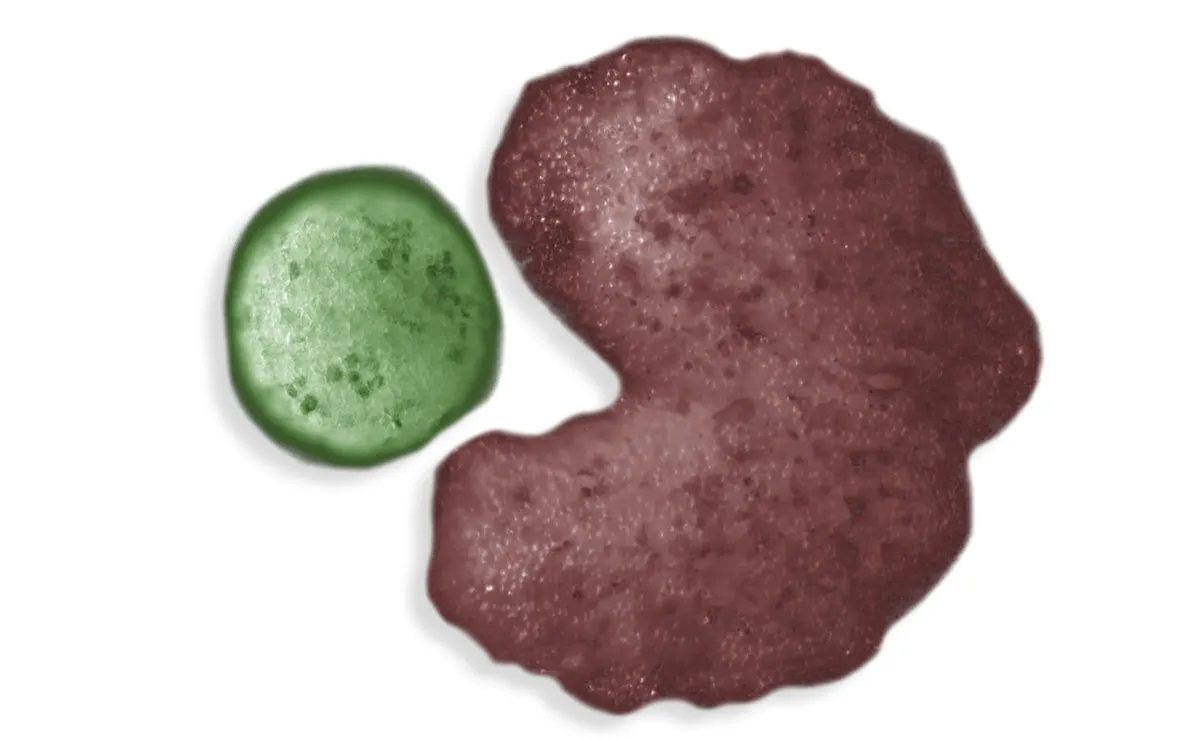
The fact that the xenobots are now capable of self-replication opens up a whole suite of potential applications, Bongard says, due to what he calls “exponential utility”.
The concept applies to any technology that does something useful and becomes more useful the further it spreads. Environmental clean-up is a good example, as well as vaccines, or technologies that could put out forest fires. These technologies don’t spread on their own, though, so they could benefit from a self-replicating carrier to help them.
Although this is all just a theory, the researchers did show through computer modelling that if they fed the xenobots enough cells and they continued to replicate, then the xenobots use for a simpler application, such as moving wires around in a circuit, would continue to grow.
If self-replicating xenobots sound like the sort of sci-fi movie scenario we ought to avoid, the thing to remember is that the parent bots can only produce offspring under certain circumstances, which, as Webster-Wood points out, the researchers control.
Without access to additional free-floating cells, they can’t replicate at all. Plus, the team’s xenobots are biodegradable and ‘die’ in a matter of days.
As Tauber puts it, “These small cellular robots are safely housed in the laboratories of Bongard’s team and could not ‘live’ in the outside world.”
In fact, ‘living’ is not a label Tauber would apply to them at all, precisely because their survival depends on such specific conditions.
Bongard, though, believes that along with other technologies – like biohybrids that combine organic and technological components – the xenobots are starting to blur the line between living and non-living, reigniting the debate about what is life.
Meanwhile, the xenobots’ behaviour has sparked other questions. For example, the researchers don’t know whether the xenobots are really cooperating when they push cells and other objects around together.
Are they able to sense each other through the millions of different receptors that exist on the surface of living cells? Or are they just mindlessly moving around like wind-up toys?
Another intriguing question, of course, is whether biological robots could also be made from human cells – and whether it’s a route the team is planning to take.
It’s certainly on the to-do list, according to Bongard. “You know, frog and human cells diverged not that long ago, when you think about the total evolutionary history of cells,” he muses, suggesting that, in principle, it should work.
Xenobots from human cells would be more compatible with medical applications, though there would be a long road to get them approved.
In the meantime, the researchers want to discover more about what it is in the frog cells’ underlying biology that makes them behave as they do.
They hope to learn how to better manipulate the living materials to create better machines. That’s something their AI is figuring out, but can’t communicate to them yet. “We’re asking the AI to make machines, but the repercussion of that is, along the way, the AI is learning more and more about biology,” says Bongard.
A key part of the work, he adds, is getting the AI to explain what it has learned about biology back to “us poor humans”.
About our experts
Prof Josh Bongard is head of the Morphology, Evolution and Cognition Laboratory at The University of Vermont. In 2007, he was awarded a Microsoft Research New Faculty Fellowship and was named one of MIT Technology Review's top 35 young innovators under 35.
Dr Falk Tauber is a postdoctoral researcher at the University of Freiburg. He is also the coordinator of the biotech livMatS demonstrator project.
Dr Doug Blackiston is a researcher at Tufts University and the Wyss Institute at Harvard University. His work has been published in journals including PloS One and the Journal Of Experimental Biology.
Read more: