There are many disagreements in the world of research, but few debates will get as heated as those surrounding animal testing. Many scientists and research advocates contend that animal experiments are crucial for learning about basic biology and disease mechanisms, and are necessary for testing the safety and efficacy of new medicines and chemicals. They point to many potent medicines that exist thanks to animal testing. Opponents, meanwhile, contend that subjecting animals to experiments for human gain is ethically unjustified. What’s more, many argue, such research is often misleading because it compares apples and oranges: results from animal studies often don’t translate to humans because the animals are just too different.
New methods
Animal welfare activists have long insisted that researchers jettison research on animals for alternative methods, such as human stem cells grown in a dish, computer modelling, or expanded clinical trials. But it’s only in the past few years that most of these tools have become truly good enough for prime-time use. Now, many researchers are embracing these alternatives. As Dr Donald Ingber, director of Harvard University’s Wyss Institute for Biologically Inspired Engineering, says, “It’s coming to a tipping point.”
Tallying the precise number of animals used in research is difficult, because countries record animal experiments differently. But estimates suggest that the count is more than 100 million animals each year worldwide. The majority are used in basic research and breeding to create specific genetic modifications. A smaller percentage of animals are used to test the effects of drugs or chemicals. More than 95 per cent of all animals used in research are mice, rats, birds and fish, but other species enter the mix, too. For example, some 60,000 monkeys like macaques are used in experiments in the US, Europe and Australia.
Read more:
- The history of medicinal drugs helps explain our relationship with them today
- Hard labour: the case for testing drugs on pregnant women
It’s hard to deny that research on animals has advanced human health. In the 19th Century, for example, French biologist Louis Pasteur used animal experiments to understand how microorganisms can cause disease, and later to develop a vaccine for rabies. Animal studies were also crucial in understanding how insulin is produced and in developing ways to supplement it in people with diabetes. Penicillin was proven effective in mice, blood transfusions were perfected in rabbits, and kidney transplants were tested in dogs and pigs.
There’s no shortage of recent examples, either. Experiments in which macaques were infected with SIV, the monkey version of the AIDS-causing HIV virus, were crucial in creating antiretroviral medicines and in developing strategies for a potential HIV vaccine. Deep brain stimulation, used by some 20,000 people with Parkinson’s disease, relied on rat and monkey models to understand how the disease affects a part of the brain called the basal ganglia and how surgically implanting a stimulator could improve patients’ motor symptoms. And brain-machine interfaces that allow paralysed people to perform everyday tasks, such as bringing a coffee cup to their lips, are being developed with the help of experiments in monkeys.
A dying breed?
Yet many scientists would now agree that for some studies, animal experiments are no longer the best way forward. “Animal testing is an important tool – it has made our world safer and it has helped to develop certain drugs – but at the same time it has very often been misleading,” says Prof Thomas Hartung, a toxicologist and the director of the Center for Alternatives to Animal Testing at Johns Hopkins University in Baltimore, Maryland. He says that in just the past few years, there has been more agreement on the limitations of animal testing and “the belief that this is some type of gold standard is fading”.
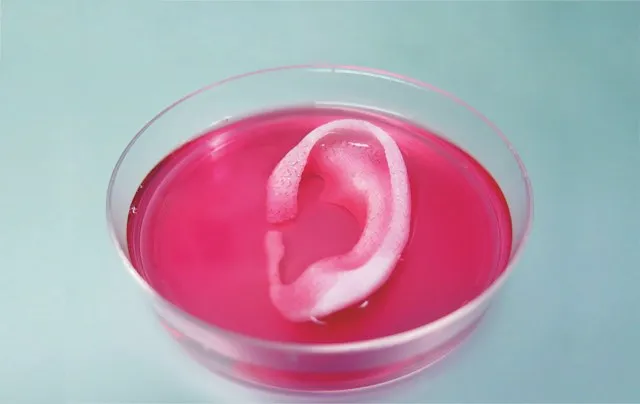
Among researchers and the public, support for limiting animal research where possible seems to be growing. In the past few years, the European Union, Israel and India have banned animal testing for cosmetics, and other countries are considering similar laws. (The UK led the way with the first such ban back in 1989.) Countries throughout the world have largely phased out research on Old World primates such as chimpanzees, and in many regions the use of other non-human primates – as well as some other mammalian species – is also on the decline. Meanwhile, regulatory bodies like the US Food and Drug Administration (FDA), which have long insisted on animal studies, are beginning to evaluate whether alternative technologies can show similar or better results, says Ingber, and companies are trying to implement these tools into their pipeline.
Changing times
It’s not just ethical concerns spurring this change. Switching to studies that use human tissue instead of animals may often make for better science. Experimental medicines that seem to be effective in animals (usually rodents) often fail in human trials; 9 out of 10 cancer drugs, and 98 out of 100 neurological and psychiatric drugs that show promise in animal tests don’t turn out to work when tested in humans. Animal studies certainly don’t deserve the full blame for this disconnect, but finding better and more predictive disease models might help, researchers say.
There are also cases where a human disease simply can’t be modelled in animals. For example, Alysson Muotri, a neuroscientist at the University of California, San Diego, studies a rare but devastating neurological disease called Aicardi-Goutieres Syndrome (AGS). The mutations causing AGS are well-known, but when Muotri studied mice that had been genetically engineered to carry these mutations, he found that they had no symptoms. When his team grew cell structures called organoids from stem cells derived from tissues of patients with the disease, they recreated the nerve cells’ glitch. They learned that what causes the disease is an immune response to an element of DNA that is specific to humans. “It’s a case where we have a truly human disorder,” Muotri says. “We couldn’t see it in the mouse, and very likely we wouldn’t see it in a primate.”
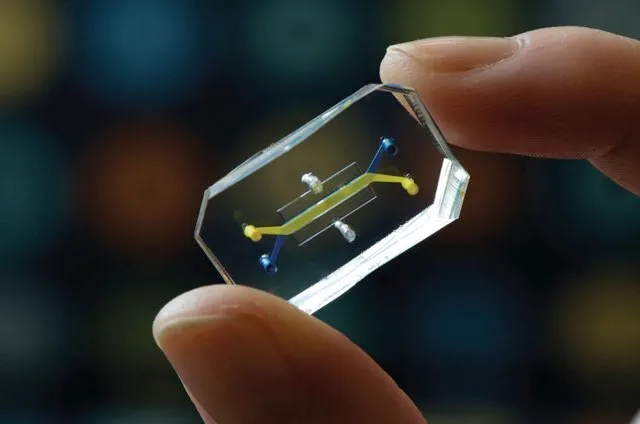
One especially promising human cell-based alternative to animal research is so-called ‘organ-on-a-chip’ technology, in which specific types of human stem cells are grown with membranes on a microchip to mimic the function of specific organs. “There are lots of things you can do on these chips that you can’t do in animal testing,” says Ingber, who has developed about 15 such devices, along with his colleagues, for mimicking the function of organs including the lungs, intestine, kidney and bone marrow. Each chip, the size of a computer memory stick, is engraved with tiny channels that are lined with human cells and artificial blood vessel tissue. The tools also capture physiological features such as blood pressure and mechanical forces that act on cells. Researchers can link up to 10 chips together with vascular channels containing human blood in order to study how organ systems interact.
“We’ve been able to mimic amazing things – diseases of all types, pulmonary oedema, asthma, chronic obstructive pulmonary disease, inflammatory bowel disease, viral infection, drug toxicities – and we’ve been able to make chips with cells from patients,” Ingber says. These devices reveal drug toxicities that don’t show up in animal models, and can also probe questions that can’t be asked in clinical trials for ethical reasons. His team is using them to model the effects of radiation exposure, as well as childhood illnesses and malnutrition.
Read more:
- Weird science: six unusual studies on our favourite livestock
- Nine unexpected effects of music on animals
But organs-on-a-chip aren’t just for university scientists. Roche Pharmaceuticals, one of the top five drug companies worldwide, embraced the technology three years ago and already uses it to test the safety of new compounds. “It opens a totally new field of opportunities to us in biology and drug discovery, and all of them are much better than an animal ever can be,” says Thomas Singer, Roche’s global head of pharmaceutical sciences. As this and other tools improve further, more companies have adopted them, banking on them being more reproducible and predictable than animal tests. “In the beginning we were very much on our own,” Singer says. “But I am convinced this technology will see a huge boost in development.”
Tiny organs
Other human cell-based alternatives to animal models are becoming available too. Prof Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine in North Carolina, is creating tissues and organs such as bladders and kidneys using a 3D printer that spits out different types of human cells. “You are miniaturising a human organ, really,” he says. Initially, his team built these organs for surgical use in the body, but he soon realised that they could be standardised and mass-produced in minutes – ideal specs for screening new medicines and testing their safety. Initially, he says, such technologies will just supplement the animal studies, but eventually they can replace them.
Toxicology studies, for medicines as well as for all sorts of other chemicals, are a low-hanging fruit for switching to alternative methods, explains Hartung. Many animal tests are particularly bad at predicting toxicity in humans, not to mention slow and expensive to conduct, and in many cases, more modern, cell- or computer-based assays have been developed. Pushing the issue, a European law passed a decade ago requires thousands of chemicals to be assessed for safety. Hartung and other toxicologists in academia and industry have developed a computer model that can predict the toxicity of a compound based on its similarity to others. “This is astonishingly powerful,” he says.
But despite the promise of all these techniques, experts say, change will probably come slowly, and it’s likely that some forms of animal models will never be eliminated at all. As Ingber puts it, “I think we are going to replace animal testing one model at a time.”
- This article was first published in February 2018
Follow Science Focus onTwitter,Facebook, Instagramand Flipboard
