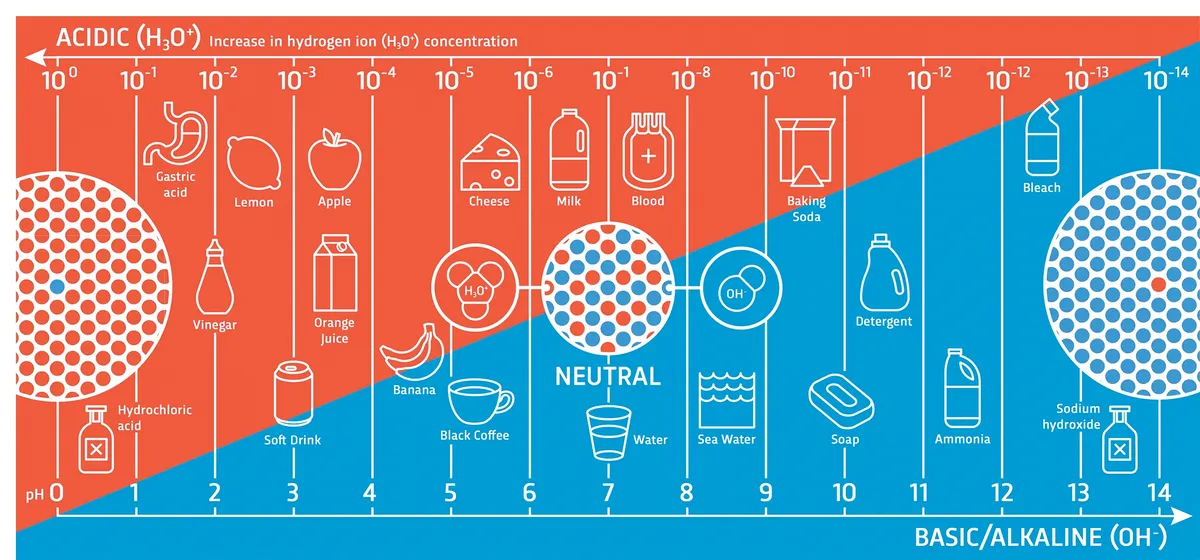
Acids are molecules that easily become negatively charged. Most acids do this by losing a proton. The stronger an acid is, the more easily it gives up a proton (hydrogen ion). Acidity is measured using the pH scale, which stands for ‘potential for hydrogen’. Each step down in the pH scale represents 10 times more hydrogen ions released. So a pH 1 acid is 100,000 times more reactive than a pH 6 acid.