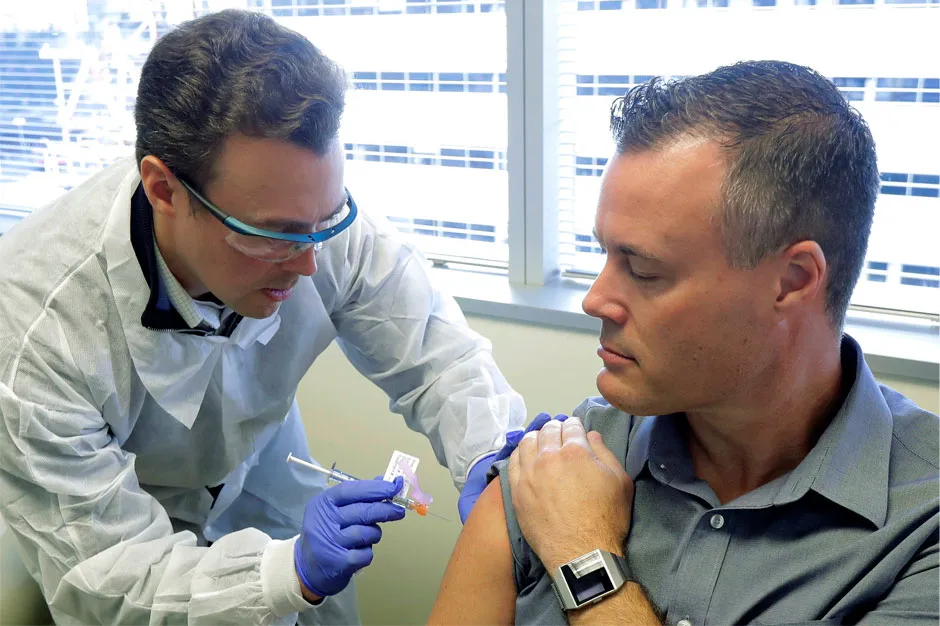
A group of volunteers in the United States have become the first to receive doses of a potential vaccine for the COVID-19 virus.Jennifer Haller, Neal Browning, and Rebecca Sirull received the first doses on Monday at the Kaiser Permanente Washington Health Research Institute in Seattle.
The volunteers – who are all healthy and young and therefore at lower risk of serious complications – will be given two injections 28 days apart to test the vaccine’s safety.

Jennifer Haller, aged 43, was the first to receive an injection of the potential vaccine.She told the Associated Press: “We all feel so helpless. This is an amazing opportunity for me to do something.”
Read more about coronavirus:
- Coronavirus: Is hand-washing really the best thing we can do to stop the spread of COVID-19?
- Coronavirus breakthrough: scientists grow virus in laboratory
- Coronavirus: aggressive 'L type' strain affecting 70 per cent of cases
Second to receive the injection out of the group of 45 volunteers was Neal Browning, while Rebecca Sirull was the third.
UK scientists are among others around the world working on a vaccine aimed at preventing outbreaks similar to the COVID-19 pandemic.
Researchers from the University of Plymouth have made progress in developing vaccines designed to prevent infections jumping from animals to humans.
A vaccine to stop COVID-19 is not expected to be available for another 12 to 18 months, with a recent Imperial College London report warning that drastic tactics to suppress the disease may need to remain in place until one is found.
How do scientists develop vaccines for new viruses?
Vaccines work by fooling our bodies into thinking that we’ve been infected by a virus. Our body mounts an immune response, and builds a memory of that virus which will enable us to fight it in the future.
Viruses and the immune system interact in complex ways, so there are many different approaches to developing an effective vaccine. The two most common types are inactivated vaccines (which use harmless viruses that have been ‘killed’, but which still activate the immune system), and attenuated vaccines (which use live viruses that have been modified so that they trigger an immune response without causing us harm).
A more recent development is recombinant vaccines, which involve genetically engineering a less harmful virus so that it includes a small part of the target virus. Our body launches an immune response to the carrier virus, but also to the target virus.
Over the past few years, this approach has been used to develop a vaccine (called rVSV-ZEBOV) against the Ebola virus. It consists of a vesicular stomatitis animal virus (which causes flu-like symptoms in humans), engineered to have an outer protein of the Zaire strain of Ebola.
Vaccines go through a huge amount of testing to check that they are safe and effective, whether there are any side effects, and what dosage levels are suitable. It usually takes years before a vaccine is commercially available.
Sometimes this is too long, and the new Ebola vaccine is being administered under ‘compassionate use’ terms: it has yet to complete all its formal testing and paperwork, but has been shown to be safe and effective. Something similar may be possible if one of the many groups around the world working on a vaccine for the new strain of coronavirus (SARS-CoV-2) is successful.
Read more: