Asked by: Paul Layfield, via email
Atoms bond with each other in order to make their arrangement of negatively-charged electrons more stable. These electrons lie in so-called ‘shells’ around the positively charged nucleus, and each shell becomes stable once it contains a certain number of electrons, as dictated by quantum theory. Bonding allows atoms to achieve this stability by swapping or sharing electrons with other atoms until each has their shells filled.
So, for example, sodium and chlorine atoms bond because the outer shell of sodium can become stable by losing an electron, while the latter can do so by gaining an electron. The two atoms are held together because losing an electron makes the sodium atom positively charged, while gaining an electron makes the chlorine atom negatively charged – and opposite charges attract.
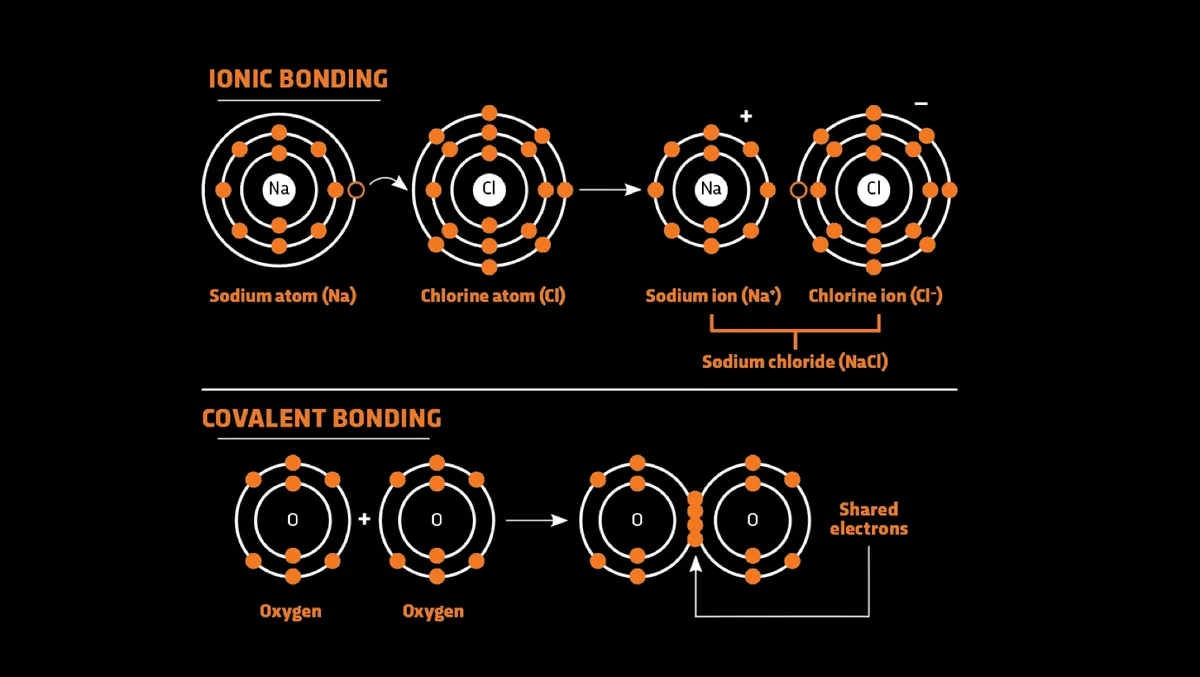
This is known as ‘ionic bonding’. Another way of bonding is to share electrons – ‘covalent bonding’. For example, oxygen atoms need two more electrons for their outer shells to become stable, and they can do this by sharing two electrons with another oxygen atom. Sharing then makes both atoms stable, and the resulting bond creates an oxygen molecule.
Read more:
- What holds together the protons and neutrons in an atom’s nucleus?
- How do two gases combine to make liquid water?