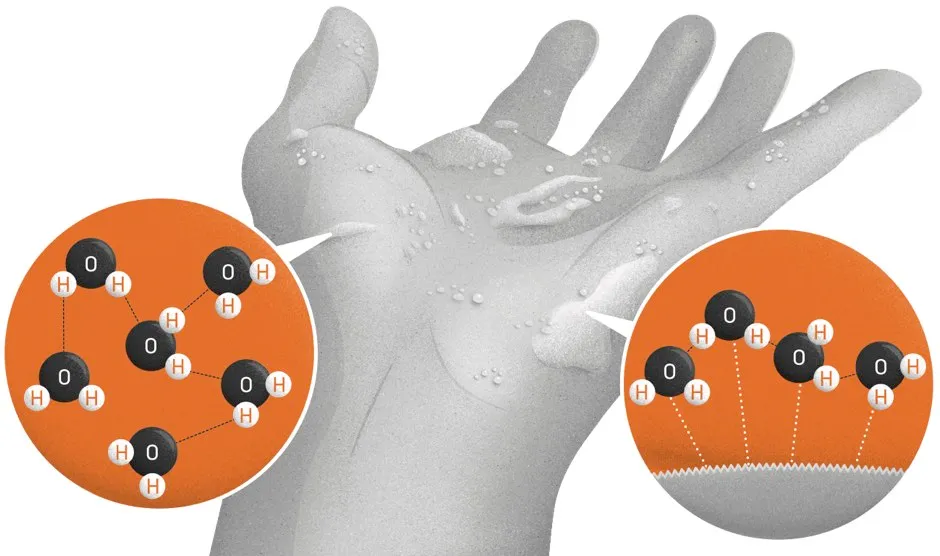
There are two effects at work. First, ‘cohesion’: the attraction of water molecules to each other. This comes from the opposing electric charges on the hydrogen and oxygen atoms of neighbouring H2O molecules. And it’s this mutual attraction of water molecules in the damp layer on the skin that creates the feeling of stickiness.
We only feel it, however, because that layer stays on the hand through the second effect: ‘adhesion’, by which water molecules stick to the surface of other substances. When your hands are soaked, there are more water molecules to adhere to the microscopic crevices in your hands, which reduces friction and makes them slippery.
Read more:
- Why does coffee make me need a poo?
- Do you get wetter if you run or walk in the rain?
- How does Blu-tack stick, but not feel sticky?
- Why does water soak upwards against gravity?