Perhaps a cold, refreshing drink on a hot day wouldn't look so appealing if the ice cubes dropped like a stone to the bottom of the glass. But why does ice float on water? In fact, why does anything float at all?
What makes something float?
An object less dense than water will float. We can explain this phenomenon with the help of a theory called Archimedes' principle.
Before you carry on, we should warn you: the following explanation will contain a lot of maths.
When you place an object in a glass of water,it will feel a buoyant force that pushes it upwards against gravity. For the object to be fully or partially underwater, some of the water must have been displaced - which you can see in the rising water level.
Archimedes' principle says that the upward buoyant force pushing against the submerged object is equal to the weight of the displaced water. An object's weight is equal to its mass multiplied by g, the acceleration due to gravity. So, the upward buoyant force – let's call it FB, for buoyant – is equal to mass of water x g.
Density is mass divided by volume, which we can rearrange to say that mass is density multiplied by volume. So we can say that FB, our buoyant force, is therefore equal to the density of water x volume of water x g.
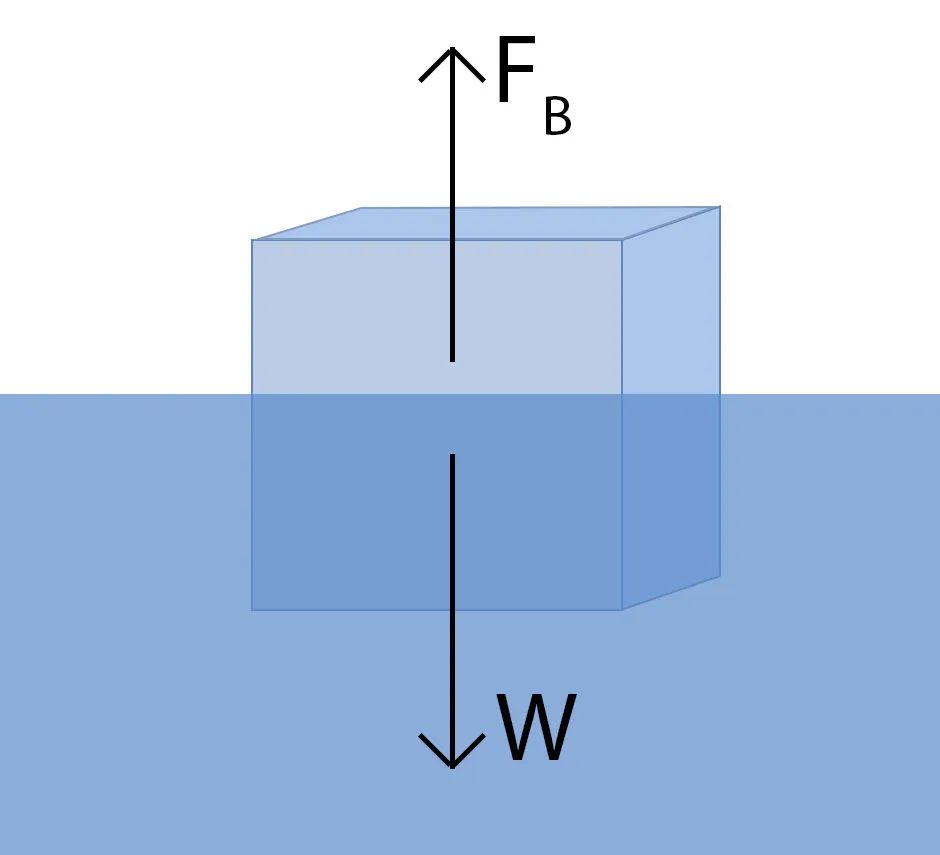
For something to float, the upward buoyant force must be at least as big as the force of gravity. So what determines whether this happens? It comes down to the realisation that made Archimedes leap out of his bath with a shout of 'Eureka!' and run naked down the street: he realised that the volume of the displaced water is equal to the volume of the object that is underwater.
So, if the volume of the water is equal to the volume of the submerged object, then our buoyant force FB is equal to the density of water x volume ofthe submerged object x g.
This buoyant force must be at least as big as the force of gravity. What is the force of gravity acting on the object? Well, that is the object's weight: the mass of the object x g. We can use the same trick as before, and say that the object's weight, which we'll call W, is equal to the density of the object x the volume of the submerged object x g.
This is almost exactly the same as the expression for FB, with one difference: the density. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water.
All of this physics comes down to a simple rule: an object will float on water if it is less dense.
What are solids more dense than liquids?
A solid will float on a liquid if it is less dense, but it's rare for the solid form of a material to be less dense than the liquid.
A material can form states of matter which differ only by the way the particles are arranged. In a solid, the molecules pack themselves tightly together into a neat, orderly repeating pattern called a crystal lattice.

When you start to heat a solid, its molecules gain energy and vibrate more strongly around their positions. Eventually, they gain enough energy that they can no longer be held in place, and they break free of the lattice. This is a liquid: the molecules are free to move around, but generally stay fairly close together.

If you continue heating it, eventually the molecules will break free from each other entirely and form a gas.
As the material goes through each phase change it becomes less dense.
Why is ice less dense than water?
If solids are denser than liquids, why does ice float on water? Because water is a special case. The molecules in water are affected by a phenomenon known as hydrogen bonding.
A water molecule is a V-shaped molecule made up of one oxygen atom in the centre with a hydrogen atom on each side. The molecule is held together by covalent bonds, which is when two atoms share a pair of electrons.
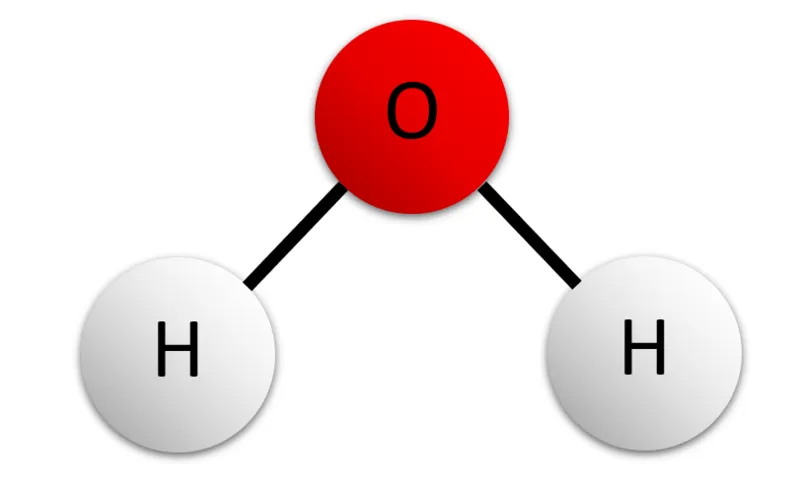
However, the oxygen atom pulls on these negatively-charged electrons much more strongly than the hydrogen atoms can. As a result, the electrons tend to hover closer to the oxygen atom than either of the hydrogen atoms. This leaves the molecule as a whole with a slight negative charge around the oxygen end, and a slight positive charge around the hydrogen end.
Since opposites attract, the slight charges on different molecules interact with each other. These interactions are called hydrogen bonds (which, technically speaking, aren't bonds at all).
In liquid form, as the molecules move around, hydrogen bonds form and break over and over, and the molecules can slip past each other.
However, as the water cools down, it starts to form into its crystal lattice structure. While the molecules want to form hydrogen bonds between the slight positive and negative charges, the same-charges repel each other, stopping the molecules from getting too close. The result is a structure that is slightly less dense than liquid water.
Is it possible to stop ice from expanding?
Water is at its densest at a temperature of approximately 4°C. If you cool it further, it begins to expand again, and once it has completely solidified into ice, it has increased in volume by about 9 per cent. The pressure exerted by this expanding ice isn’t infinite but it is enormous.
The bulk modulus of ice is around 8.8 x 109 pascals. This means that if you seal a full container of water and freeze it, the pressure on the sides of the container will be approximately 790 megapascals or 114,000 pounds per square inch. That’s 7,800 atmospheres and according to Professor Martin Chaplin of London South Bank University, the world’s leading expert on the properties of this bizarre substance, there’s no material on Earth capable of withstanding the pressures generated. –Robert Matthews
Without room to expand, would water still freeze?
If you put water in a very strong, rigid container and continue to cool it, the pressure will begin to rise as more and more molecules adopt the lattice formation and press against the remaining molecules still in the free liquid state. If the container doesn’t break, the pressure will rise very fast until eventually at around 200 megapascals (roughly 2000 Atmospheres), the atoms begin to rearrange again into a new, more compact configuration.
There are 13 known forms of ice that are stable at different temperatures and pressure. Ordinary ice is called ice Ih, whereas the most dense of the high pressure varieties is ice III. In an enclosed container, the expansion pressure will reach an equilibrium point and the water will freeze as a mixture of ice Ih and ice III. – Keiron Allen
Read more: