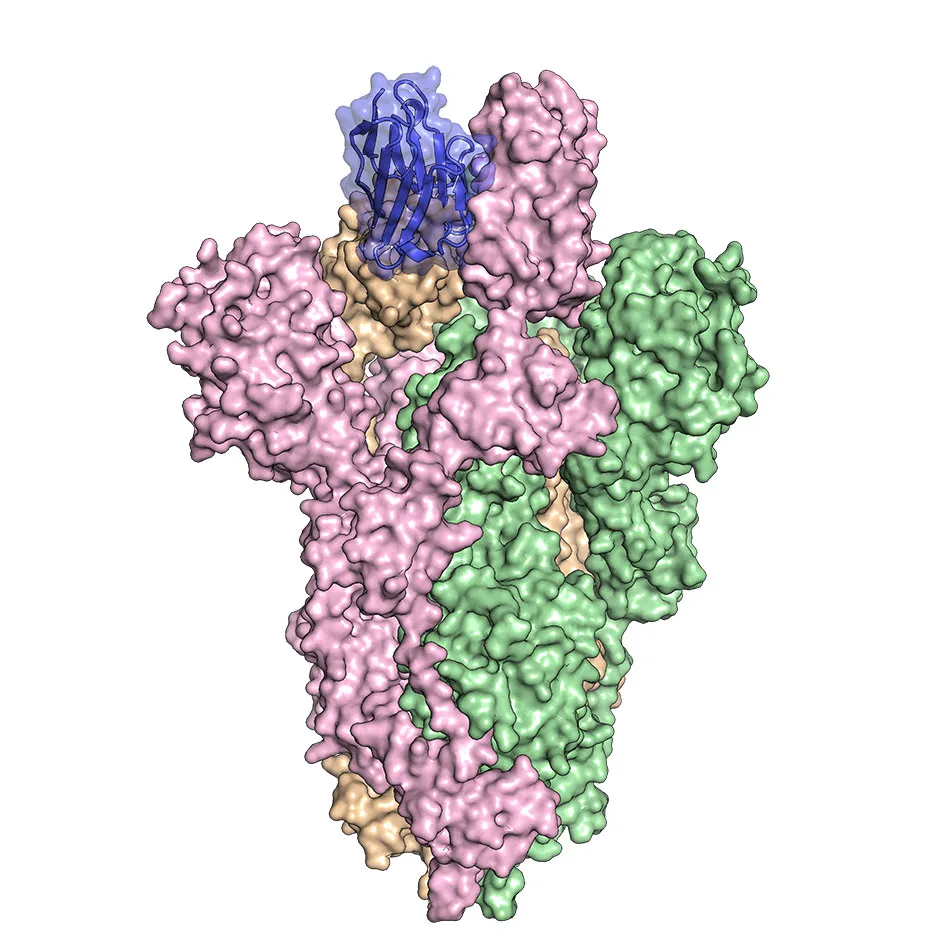
Researchers at the McLellen Lab in Austin, Texas, are developing a potential treatment for the SARS-CoV-2 virus based on antibodies found in llamas. The antibodies can be used to attack specialised ‘spike proteins’ found on the surface of the SARS-CoV-2 virus and prevent it from entering human cells.
We spoke to Daniel Wrapp, a PhD candidate in the McLellan Lab.
What is a spike protein is and what is its function?
A spike protein is a protein that decorates the surface of a coronavirus. It functions to attach that virus to a host cell by binding to a receptor. Once it's attached, it fuses the viral membrane with the host cell membrane so that the virus can enter into the host cell and begin the process of replication.
The spike protein is the main machine that the virus uses to enter into our cells. So, without the spike functioning properly on the surface of a virus, the virus is neutralised and is non-infectious.
Why did you focus your work on llamas?
Camelids, which is a family that includes camels, llamas, alpacas and a couple other animals, produce this special class of small antibodies, which are sometimes called nanobodies.
They're about half the size of the conventional antibodies that you or I would produce, and because of that small size, they have enhanced stability and they're also capable of binding to small crevices or pockets that larger antibodies wouldn't otherwise be able to bind.
Read more about the coronavirus:
- 'No evidence' of coronavirus mutating into more dangerous strains
- Coronavirus vaccine: could genetic material help defeat COVID-19?
Your work initially started by looking at SARS-CoV-1.
We started this in 2016, so well before the current pandemic hit and we immunised a llama with these spike proteins from the MERS coronavirus and the SARS-CoV-1 coronavirus.
We were actually writing up these results in a manuscript to submit when the pandemic broke. So we looked at the genome sequence that was released by the Chinese CDC and we compared it to the sequence of the SARS-CoV-1 spike protein.
We knew based on our research that the site that this antibody bound was well conserved between the two viruses. So, we took the time to actually test a binding of our antibody to the SARS-CoV-2 spike we found that it bound with fairly high affinity.

Then we performed some studies in cell culture to show that this antibody also prevented infection by SARS-CoV-2. Also, our lab was the first to produce the atomic structure of the SARS-CoV-2 spike protein, that was published in Science in early February.
So, we had all of these reagents already prepared from our previous experimentation and we had the antibody. It was sort of a no brainer for us to test the binding.
Have you started any pre-clinical trials?
Yes, we have. We're doing this work in collaboration with some researchers from Ghent University in Belgium, and they've begun pre-clinical trials which are taking place in hamsters.
Read more about treatments for COVID-19:
- Coronavirus: supercomputers drafted in to detect potential treatments
- Coronavirus: UK patients to test existing drugs as COVID-19 treatments
What sort of progress have they made?
We haven’t actually challenged the hamsters yet because there are so many difficulties associated with the bio-safety of infecting animals with live virus.
But they're they're moving as quickly as they can to get it into hamsters and then if everything goes well in hamsters, organise non-human primate trials so that we can eventually move into humans, hopefully.
So typically, what sort of timeframe would be looking at in the best-case scenario?
We're hoping for about a year until we would have an approved product that could be put into humans for treatment.
How would we administer this antibody treatment to a human patient?
It would be administered much like any other conventional antibody treatment, by injection. Really, the nice thing about an antibody treatment as opposed to a vaccine is that you could administer it to people who are already infected, and it would rapidly begin to reduce the disease burden and hopefully reduce the severity of the symptoms.
Can you explain the difference between an antibody treatment and a vaccine?
A vaccine has to be administered probably about two months before exposure to the infectious agent in order to be effective. That's because you want to present the human immune system with something that looks like a pathogen so that it can raise its own antibodies and fight off infection if it's ever presented with that pathogen infection scenario.
Unfortunately, that's sort of useless if you've already been infected. So in that case, you have an antibody treatment for somebody who is already infected to help them get better more quickly.
Could the treatment also potentially be used as preventative measures in, for example, key workers?
Yes, I'm glad you brought up essential workers because people like health care workers could be administered this drug and then it would reduce or prevent their susceptibility to infection when treating patients.