Antibodies derived from llamas have been shown to combat coronavirus in laboratory tests.
Researchers hope the antibodies – known as nanobodies due to their small size – could eventually be developed as a treatment for patients with severe COVID-19.
The immune system produces antibodies when it is being attacked, or in response to infections. Llamas, camels and alpacas naturally produce quantities of small antibodies with a simpler structure, that can be turned into nanobodies.
Read more about coronavirus antibodies:
- Coronavirus: antibody immunity could last 'just months'
- Male donor plasma 'contains more coronavirus antibodies' than female
The team from the Rosalind Franklin Institute, Oxford University, Diamond Light Source and Public Health England engineered their new nanobodies using a collection of antibodies taken from llama blood cells.
They found that the nanobodies bind tightly to the spike protein of the SARS-CoV-2 virus (the virus that causes COVID-19), blocking it from entering human cells and stopping infection.
In the study published in Nature Structural and Molecular Biology, the team also identified that the nanobodies bind to the spike protein in a new and different way to other antibodies already discovered.
Similar research into llama antibodies as a treatment for COVID-19 is underway at the McLellan lab in Austin, Texas.

James Naismith, director of the Rosalind Franklin Institute and professor of structural biology at Oxford University, said: “These nanobodies have the potential to be used in a similar way to convalescent serum, effectively stopping progression of the virus in patients who are ill.
“We were able to combine one of the nanobodies with a human antibody and show the combination was even more powerful than either alone. Combinations are particularly useful since the virus has to change multiple things at the same time to escape – this is very hard for the virus to do.
“The nanobodies also have potential as a powerful diagnostic.”
Professor Ray Owensfrom Oxford University, who leads the nanobody programme at the Franklin, said the researchers are hopeful they can push the breakthrough on into pre-clinical trials.
Read more about treatments for COVID-19:
- Coronavirus: simple salt water solution 'could help reduce symptoms'
- Coronavirus blood trial targets hormone imbalance
Professor David Stuart, from Diamond Light Source and Oxford University, said: “The electron microscopy structures showed us that the three nanobodies can bind to the virus spike, essentially covering up the portions that the virus uses to enter human cells.”
Researchers started from a lab-based library of llama antibodies, and are now screening antibodies from Fifi, one of the ‘Franklin llamas’ based at the University of Reading, taken after she was immunised with harmless purified virus proteins.
The team is looking at preliminary results which show that Fifi’s immune system has produced different antibodies from those already identified, which will enable cocktails of nanobodies to be tested against the virus.
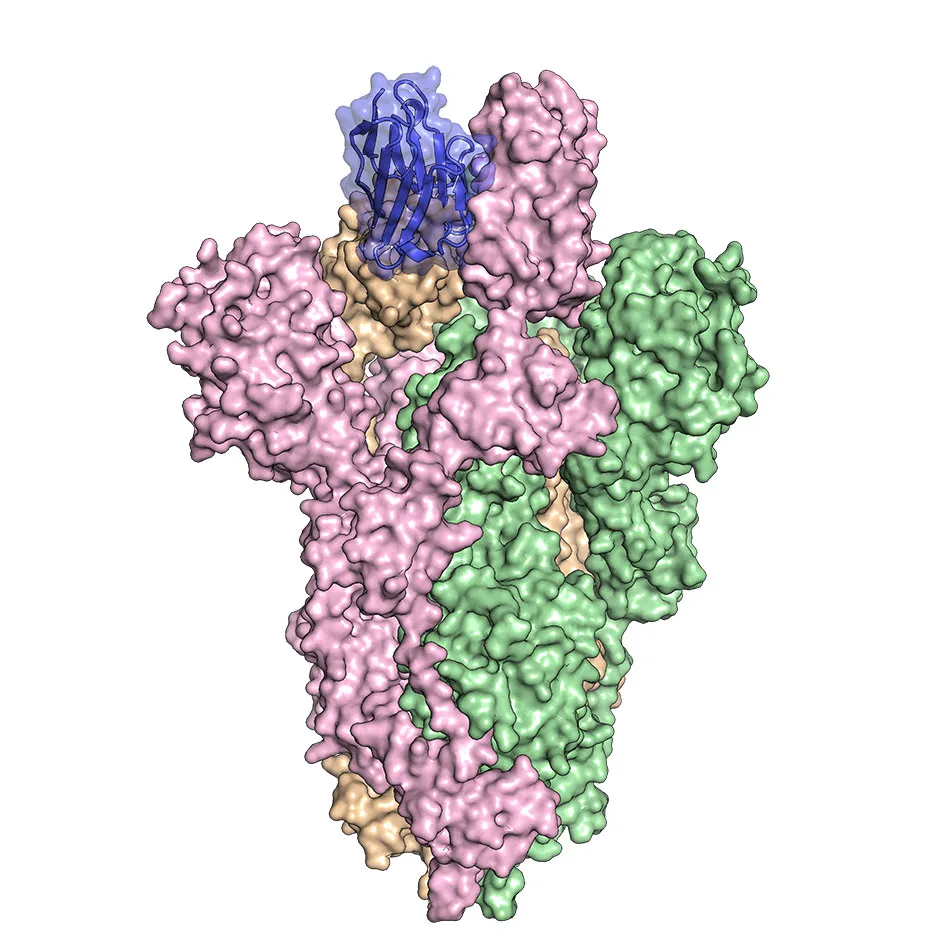
In a separate study published in Nature Medicine, scientists say they have uncovered how a crucial component of the immune system responds to the spike protein of SARS-CoV-2.
Coronavirus particles have a corona (crown) of proteins that resemble spikes, which enable the virus to attach and enter cells in humans.The spike protein is crucial in inducing neutralising antibodies to protect from reinfection.Neutralising antibodies not only bind to the viral spike protein but prevent it from being able to attach to and enter human cells.
Researchers from the Peter Doherty Institute for Infection and Immunity (Doherty Institute) investigated how the immune system, particularly B and T cells, responds to the spike.
B cells are responsible for producing the antibodies that recognise SARS-CoV-2, while T cells play an important role in supporting the development of the B cell response.
Read more coronavirus news:
- Coronavirus may have ‘devastating impact’ on the heart
- COVID-19 vaccine ready in first half of 2021 if trials go 'really well'
Dr Jennifer Juno, from the University of Melbourne – and a postdoctoral researcher at the Doherty Institute, said they looked at people who had recovered from COVID-19 who had mostly experienced mild or no symptoms.
She said: “We found that those who showed strong neutralising antibody activity had a robust B cell response, but most surprisingly, we also found that a particular subset of T cells, called T-follicular helper cells, was a great predictor of an effective immune response.”
She added: “Now we know how the immune system responds to the spike protein, and we have these biomarkers, or predictors of what elicits a good or poor immune response to COVID-19, we can look at the vaccine candidates and see what will offer the best protection.”
How do scientists develop vaccines for new viruses?
Vaccines work by fooling our bodies into thinking that we’ve been infected by a virus. Our body mounts an immune response, and builds a memory of that virus which will enable us to fight it in the future.
Viruses and the immune system interact in complex ways, so there are many different approaches to developing an effective vaccine. The two most common types are inactivated vaccines (which use harmless viruses that have been ‘killed’, but which still activate the immune system), and attenuated vaccines (which use live viruses that have been modified so that they trigger an immune response without causing us harm).
A more recent development is recombinant vaccines, which involve genetically engineering a less harmful virus so that it includes a small part of the target virus. Our body launches an immune response to the carrier virus, but also to the target virus.
Over the past few years, this approach has been used to develop a vaccine (called rVSV-ZEBOV) against the Ebola virus. It consists of a vesicular stomatitis animal virus (which causes flu-like symptoms in humans), engineered to have an outer protein of the Zaire strain of Ebola.
Vaccines go through a huge amount of testing to check that they are safe and effective, whether there are any side effects, and what dosage levels are suitable. It usually takes years before a vaccine is commercially available.
Sometimes this is too long, and the new Ebola vaccine is being administered under ‘compassionate use’ terms: it has yet to complete all its formal testing and paperwork, but has been shown to be safe and effective. Something similar may be possible if one of the many groups around the world working on a vaccine for the new strain of coronavirus (SARS-CoV-2) is successful.
Read more:
