Oxygen is a word familiar to us all, but few realise its name is based on an error. In fact, it is one of a few element names which derives from a case of mistaken identity. Dr Peter Wothers, a Teaching Fellow in the Department of Chemistry at the University of Cambridge reveals some of the chemical elements suffering from a case of mistaken identity.
Oxygen
For many years it was thought that things burned because they contained some flammable principle named phlogiston; the more phlogiston something contained, the better it could burn.
The phlogiston was supposedly lost during the combustion and given up to the air. When Joseph Priestley first isolated the gas we call oxygen, he named it dephlogisticated air, since he thought things burned so readily in it because the gas could receive all the phlogiston given out by the burning substance.
In contrast, nothing could burn in the gas we call nitrogen since this was fully saturated with phlogiston – hence he called that gas phlogisticated air.
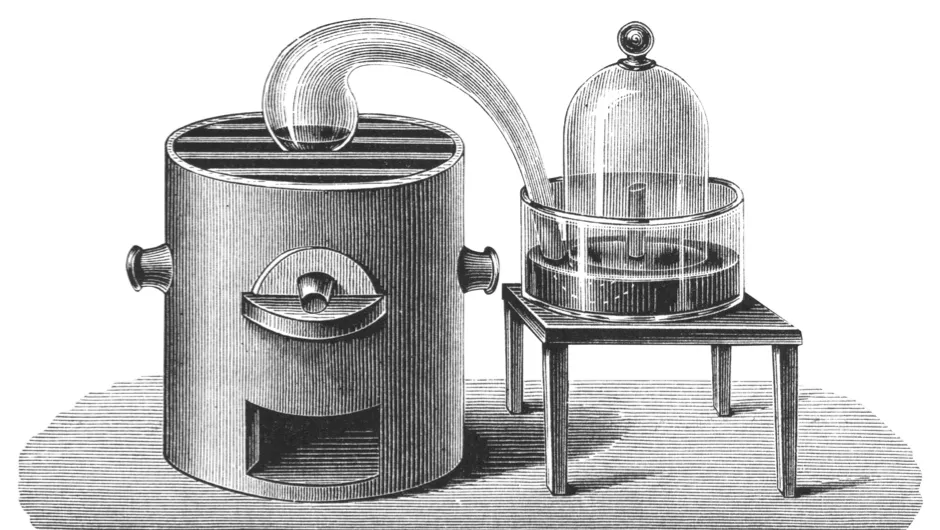
The first to correctly understand that things burned by combining with part of the air (the oxygen) was the French chemist Antoine Lavoisier.
Initially, he referred to the gas as vital air but he actually thought this was itself made up a still more basic component combined with heat. He called this component oxygen, which he derived from the Greek words meaning ‘begetter of acids’, since he thought it was the essential principle of all acids.
There was some sense in this; when sulfur burns in oxygen and the resultant products dissolved in water, an acid is formed. Similarly, when phosphorus or carbon are burned.
Read more about oxygen:
- What’s the longest an animal can survive without oxygen?
- Could alien life breathe a gas other than oxygen?
- Oldest oxygen in the Universe discovered
- How many trees does it take to produce oxygen for one person?
But what Lavoisier missed was the crucial role of the water – we would now say that the hydrogen ions that ultimately come from the water are the principal of acidity; indeed, the ‘H’ in pH, the scale used for measuring acidity, stands for hydrogen ions.
Hydrochloric acid is a strong acid which contains no oxygen but nonetheless gives high concentrations of hydrogen ions when dissolved in water. Given that acids all produce hydrogen ions, perhaps the element hydrogen should have received the name ‘begetter of acids’ – oxygen.
But then, what name for oxygen? There could be none more appropriate than the name hydrogen, a name also created by Lavoisier for the element that forms water.
However, even though there are two atoms of hydrogen in a water molecule and only one atom of oxygen, because the atom of hydrogen is so light, it only makes up about 10 per cent of the mass of water; the bulk of the mass comes from the heavier oxygen atom.
As soon as Lavoisier proposed the name ‘hydrogen’ it was suggested that the name was more appropriate for the element that makes up the bulk of the mass of water. So it would be far more sensible if the names hydrogen and oxygen were swapped around: H2O would become O2H.
Molybdenum
Oxygen is not the only element named inappropriately – molybdenum also ended up with its name through a misunderstanding. This time it was a mix up with three substances that had a rather similar appearance – a mineral form of lead sulfide called galena, the mineral molybdenite, and naturally occurring graphite. All three of these are shiny, dark grey solids.
By far the most common of the three is what we would now call galena, lead sulfide, so it is not surprising that the less common minerals were confused with this and ended up with names related to lead species.
The 16th-Century mining authority Agricola, who in 1556 published the beautifully illustrated De Re Metallica (On the nature of metals), mentions a ‘sterile’ variety of galena - one that is completely consumed by the fire, yielding no lead. This is most likely a reference to graphite, which when heated strongly in air forms gaseous carbon dioxide and leaves only a residue of its trace impurities.
Read more about the elements:
- The weird ways extraordinary scientists made synthetic elements
- It’s elemental: how to become a periodic table pub quiz champion
- Which elements are in danger of running out?
- The first unambiguous descriptions of graphite are from the late 16th Century, when its use by artists and writers is noted. Particularly famous was the English graphite from the Borrowdale mine near Keswick in Cumberland, and it was probably a sample from here that was referred to in the first description and illustration of a pencil in 1565.
The mines were included in the 1610 English edition of William Camden’s Britain, or, to give it its full title, Britain, or A Chorographicall Description of the Most Flourishing Kingdomes, England, Scotland, and Ireland, and the Ilands Adioyning, out of the Depth of Antiquitie. Here the author describes where:
‘…the river Derwent hideth himselfe in the Ocean; which having his first beginning in Borrodale, a valley hemmed in with crooked hilles, creepeth betweene the mountains called Derwent Fels’. [In addition to copper mines,] ‘here also is commonly found that mineral kind of earth or hardned glittering stone (we cal[l] it Black-lead) with which painters use to draw their lin[e]s & make pictures of one colour in their first draughts’.
Not surprisingly, Camden is not sure exactly what the mineral is, and is content to ‘let others for me search it out.’
The term ‘black lead’ had earlier been used for the metallic lead itself but here it is used for a mineral which is distinctly lead-free but, like its namesake, could easily be used to mark paper - or indeed sheep, as the locals from Borrowdale were wont to do.
Graphite is still sometimes called plumbago, a name more correctly applied to ores of lead (our word plumber and the chemical symbol Pb both derive from the Latin word for lead, plumbum.)

Another term occasionally used for mineral graphite was molybdæna, which derives from the Greek word for lead, molybdos (μόλυβδος). But molybdæna was also used for a rarer mineral we would now called molybdenite, a substance remarkably similar to graphite with the same lubricating properties and ability to mark fingers, paper, or, presumably, sheep.
The first to recognise the difference between graphite, molybdenite and galena was the Swedish master chemist Carl Wilhelm Scheele, who in 1778 and 1779 published two papers clearly identifying that graphite was a form of carbon, whereas molybdæna contained a new element which he isolated as its hydrated oxide and referred to as acid of molybdæna.
A couple of years later, a mineralogist friend of Scheele prepared the metallic element from the mineral – ‘perfect molybdænic metal’ which he called molybdænum. Hence molybdenum, an element now used in thousands of tonnes each year to harden steel, is actually named after the Greek for a different element, lead.
Antimony, Gold, and Jupiter's Wolf: How the elements were named by Dr Peter Wothers is out now (£20, Oxford University Press)
