Most conventional electric vehicles and mobile phones use lithium-ion batteries, which have an electrolytegel inside them to separatethe positively charged graphite anode fromthe negatively chargedlithium cathode.
These are relatively cheap to make but suffer from thermal runaway – heat them beyond a specific temperature and an unstoppable chain reaction causes them to disintegrate in fiery explosions.
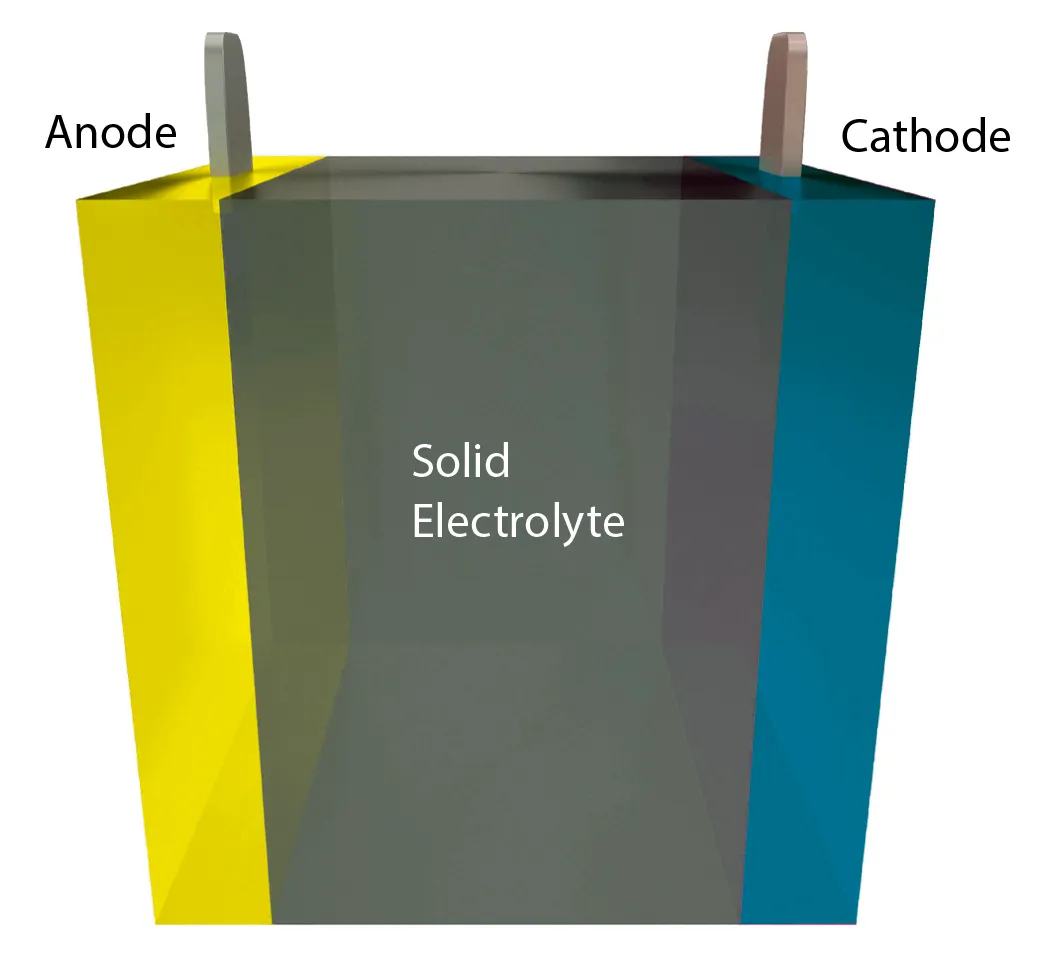
Solid-state batteries replace the electrolyte gel with a solid material such as ceramic or glass, which makes them less flammable, faster charging, lighter, and higher power.
At present, they’re still under development and remain costly to manufacture. This maysoonchange,as companies are spending billions on the development of thisnew technology.
Read more:
- Why do batteries explode?
- How are batteries recycled?
- How do lithium-ion batteries self-extinguish?
- Why can’t you recharge batteries instantly?
Asked by: John Awbery, via email
To submit your questions email us at questions@sciencefocus.com (don't forget to include your name and location)
- This article first appeared inissue 374ofBBC Science Focus Magazine–find out how to subscribe here
