It’s one of the most commonly used materials on the planet – found in everything from pencils to nuclear reactors – but why does graphite conduct electricity?
Answer: the very reason why metals do. “Metals conduct electricity as they have free electrons that act as charge carriers. Graphite is just the same,” says Dr Dong Liu, physics lecturer at the University of Bristol.
As she points out, graphite is made from carbon atoms, which have four electrons in their outer shells. While three of these form a strong bond with other atoms, one electron is left free (and known as ‘delocalised’) in graphite.
Importantly, the layers in graphite are 'aromatic'. Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds (see diagram below). This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers.
“You can think of electricity as like a motorway flow,” adds Liu.
“The free electrons are like cars travelling from one end of the material to the other, carrying charge. In other materials, there may not be free cars to make this journey, meaning they're not conductive."
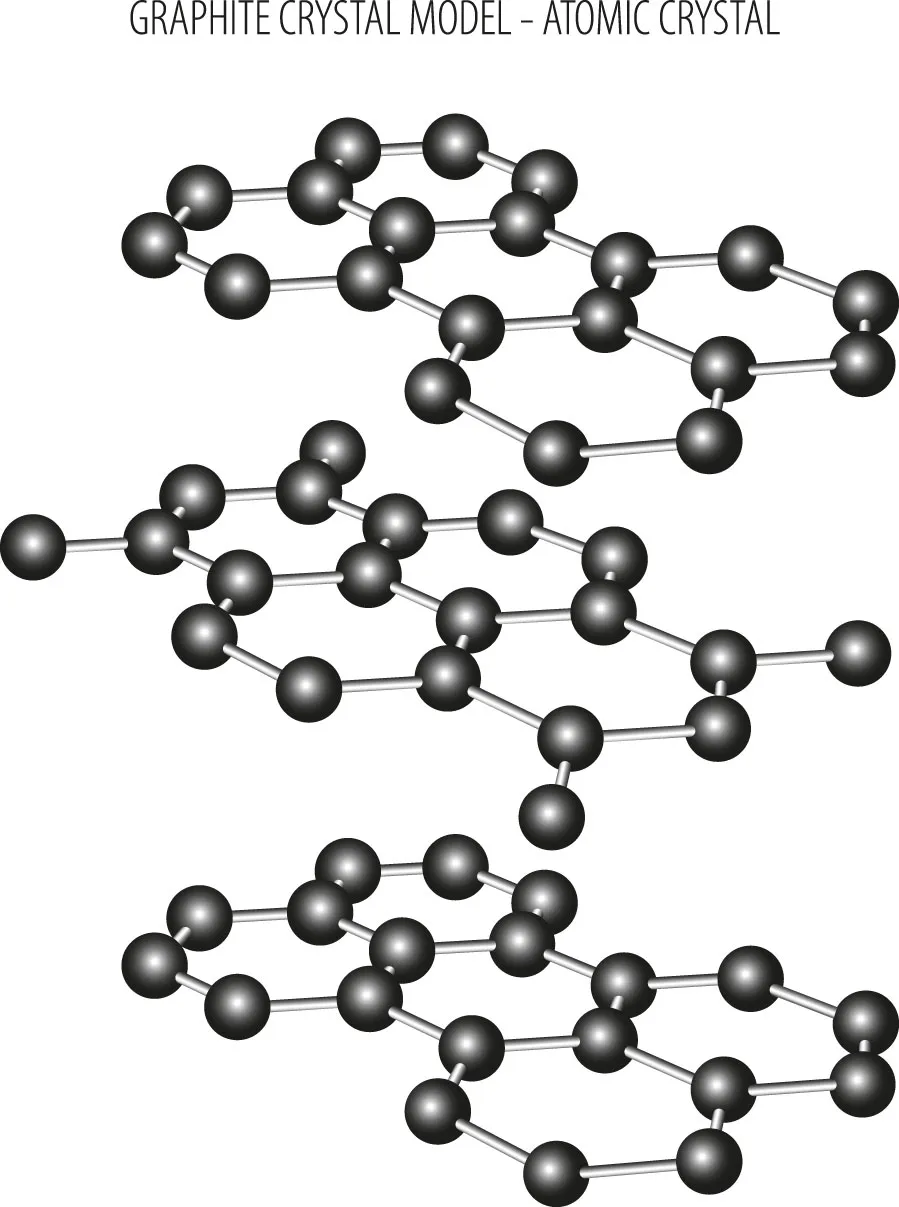
Why can graphite conduct electricity but diamonds can’t?
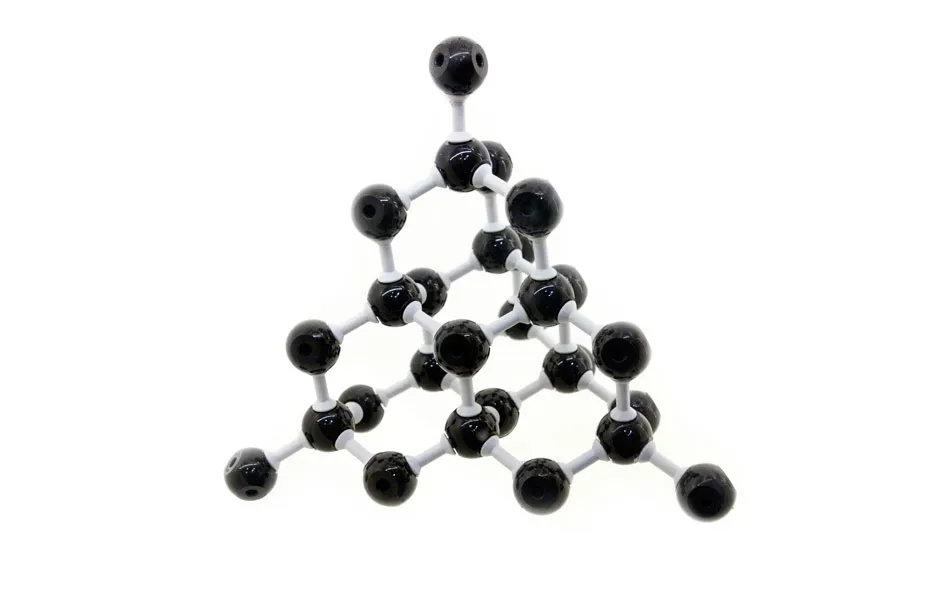
True, both diamonds and graphite are made from carbon. However, their structures are significantly different. Remember how graphite carbon atoms have a free electron? The same isn’t true for diamond: all four electrons have formed strong single bonds with other atoms.
“It’s all those covalent bonds that make diamond so hard – the hardest material,” says Liu. “There are no free cars driving on its highway – no electrons to pass charge between point A and point B."
Why does graphite have a high melting point?
Both diamonds and graphite have extremely high melting points – both above 3000 degrees Celcius.
As you might have guessed, this is due to the strong covalent bonds between their atoms.
“When you’re melting something, you’re increasing the distance between atoms,” says Liu.
“You need a lot of energy to break the bonds in graphite’s layers. Between the layers of atoms the bonds are weak, but the layers themselves are strong.”
Read more:
- Have pencils ever contained lead?
- Element in the room: chemical elements with mistakes in their names
This is why approximately 6,000 pounds of graphite is used at 14 nuclear reactors across the UK.
“Unlike metals, graphite isn’t going to melt easily. At 1000 degrees Celcius, most metals will become floppy. Graphite won’t, but it will slow the fast-moving neutrons caused a nuclear chain reaction,” says Liu.
“Graphite is also better than diamond for a nuclear reaction because if there’s a tiny flaw in the diamond – a small crack – it will break when strained. Diamond is hard, but very brittle.
"Plus, It’s very difficult to obtain a huge block of diamond. Sure, you could turn a lot of graphite into diamond, but that’s very difficult.”
Difficult is an understatement. To turn graphite into diamond you need an extremely high temperature – above 2000 degrees Celcius – and pressure beyond 100 kbar (nearly 1000 times of our normal atmosphere pressure).
In short: graphite trumps diamond. We still don't recommend proposing with a graphite ring, though.
About the expert in this piece
Dr Dong Liu is a physics lecturer at the University of Bristol. Her current research focuses on building materials to be used in the safe operation of nuclear reactors.
Read more: