A baby born in Mexico is the first to have been conceived using a new “three person” fertility technique, which was approved for use in the UK back in February 2015. Parliament voted to amend the 2008 Human Fertilisation and Embryology Act to allow ‘three-parent IVF’ for families that carry mitochondrial diseases, which are coded in the genes and are passed from mum to child via the mitochondria, the ‘batteries’ of the cell.
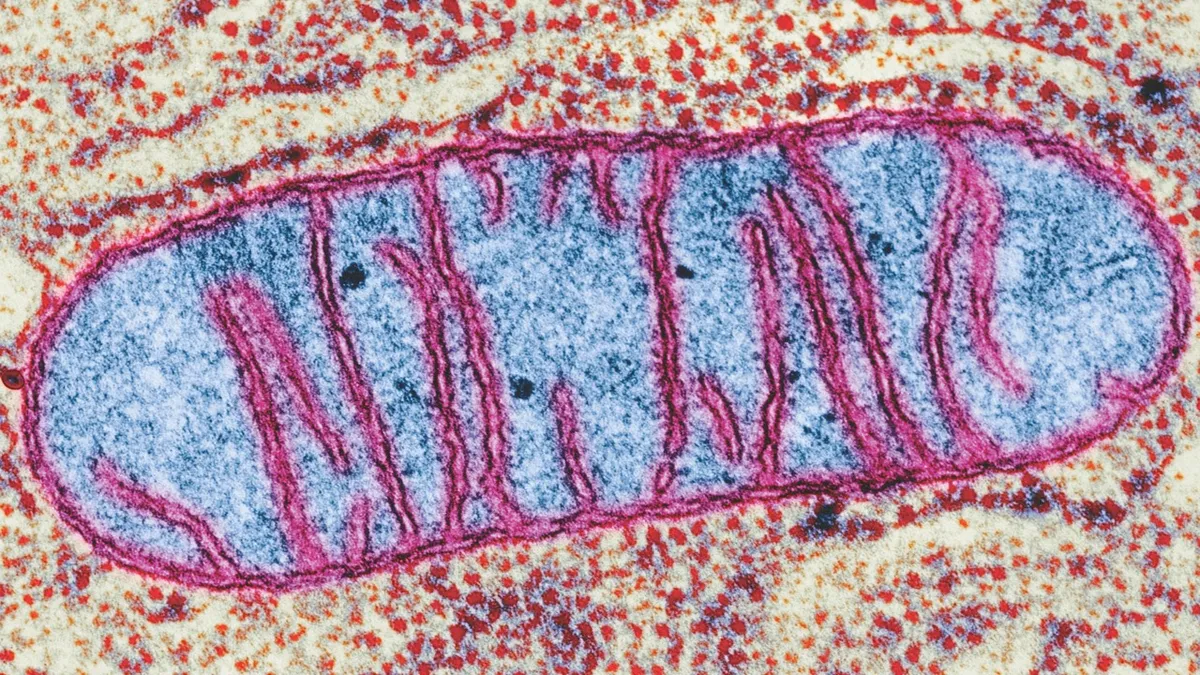
Mitochondria are tiny disc-shaped organelles (minuscule organs) carried within cells. The primary function of mitochondria is to produce ATP, the biological currency of energy. The number of mitochondria varies widely between cell types: red blood cells don’t contain any, but liver cells can hold up to 2,000 each.
Human egg cells contain mitochondria the way most cells do, but sperm cells only have them in their tails. During fertilisation, the head of the sperm, which contains its genes, is inserted into the egg. The tail of the sperm – and therefore its mitochondria – is left behind. This is why all of us only inherit our mitochrondrial DNA from our mothers.
Malfunctioning mitochondria can produce a wide variety of illnesses for which we have no cure. They regularly strike the organs that have the greatest energetic demands, including the kidneys, heart, liver, brain, muscles and central nervous system. Mitochondrial conditions are often fatal in infancy, but can frequently strike in adolescence or adulthood. It is estimated that one in 200 children in the UK carries some form of genetic mutation that could lead to mitochondrial disease at some point in life. Every year, one in 6,500 babies is born with a mitochondrial condition so severe that they will not reach adulthood – or even their first birthday.
“Mitochondrial diseases are horrible and cruel – especially because as a parent there is nothing you can do,” says Liz Curtis, whose daughter Lily died at eight months old from Leigh Syndrome. While Lily died when she young, others live for five or 10 years, slowly deteriorating. “To watch a child lose the ability to walk, talk, eat and eventually smile is crushing,” says Curtis. She set up the Lily Foundation in her daughter’s honour to support the families of children coping with mitochondrial conditions and to fund research into potential cures – because none were available to prevent Lily’s death.
Silent killer
Currently in the UK, more than 150 children a year are born who will suffer severe mitochondrial disease – often unbeknownst to them or their families. And new research hints that mitochondrial anomalies may play a role in the diseases of old age, such as prostate cancer and Alzheimer’s. Curtis, like most parents, had no idea she carried any gene that was faulty. “I’d never even heard of mitochondrial disease, nobody in my family had. It came completely out of the blue,” she says.
The main reason people like Curtis can carry a mitochondrial mutation, but not exhibit symptoms themselves, is due to a quirk of mitochondria called ‘heteroplasmy’. While the DNA in the nucleus of every single non-sex cell in the human body is identical, the selection of mitochondrial genes varies.
When one cell divides, its chromosomes are duplicated; each daughter cell receives identical chromosomes. But the tiny mitochondria – remember, there can be up to 2,000 of them per cell – are split randomly between the two daughter cells. Which cell gets which mitochondria carrying which genes is a matter of chance. This is why one sibling in a family may inherit a mitochondrial disease and one will not, and why a mother can unknowingly carry a dangerous gene.
Mutations that can lead to disease are therefore scattered randomly and unevenly between different cells. Disease-causing mitochondrial mutations vary not just between individuals, but between tissue types in one person: we are all mitochondrial mosaics. A certain ‘threshold’ amount of a malfunctioning mitochondrial gene in any given cell needs to be reached for an illness to manifest.
Altered embryos
The technique that was legalised in the UK at the beginning of 2015 will allow a mother to give birth to a baby that is genetically hers, but there will not be the risk of it inheriting mitochondria with dangerous mutations. The process is known as ‘mitochondrial donation’ or ‘mitochondrial transfer’. A mother-to-be carrying faulty mitochondria can opt to have her nuclear DNA removed from her eggs and implanted into a donor egg carrying healthy mitochondria. The egg is then fertilised with sperm from the father before being implanted into the mother’s uterus for pregnancy to continue as usual.
A recent study from the Wellcome Trust Centre for Mitochondrial Research at Newcastle University estimated that 2,473 women in the UK are at risk of passing a mitochondrial disease to their children and thus could benefit from the treatment.
“I’m over the moon that the law was changed. It’s hugely rewarding to know that families can have their own child that will be free from disease,” says Curtis.
Three parents?
Children who would be conceived in this way have been dubbed ‘three-parent babies’ by the press, as they technically carry DNA from three people (albeit just 37 genes from the donor egg, compared to 20,000 from the mother).
“It’s unfortunate that the ‘three-parent baby’ term was coined,” says Shamima Rahman, a Professor of Paediatric Metabolic Medicine at the UCL Institute of Child Health, who began working with mitochondrial diseases 20 years ago. “I was very concerned that we were seeing a group of disorders that nobody really knew anything about, much less how to treat. They can be extremely debilitating and it’s heartbreaking for the parents.”
Aside from sensationalism, the ‘three-parent’ nickname is misleading, in several respects:
One, the female mitochondrial donor is not likely to have any role whatsoever in bringing up the child. Two, the amount of DNA carried in the mitochondria – just 37 genes in total, compared to 20,000 in the nucleus – is tiny, a mere 0.1 per cent of the entire genome. And three, children have already been born who carry DNA from three parents.
Women who act as surrogate mothers have been found to pass minute amounts of mitochondrial DNA to the babies they carry for nine months. Meanwhile, in the late 1990s, children conceived through ‘ooplasmic transfer’ – an IVF technique used to bolster the viability of eggs by injecting cytoplasm from young donor eggs into the older eggs of women undergoing fertility treatment – were later found to carry small amounts of DNA from the donor. Some of the resulting children are alive and well. The US Food and Drug Administration (FDA), however, put the brakes on this treatment back in 2001, and has yet to approve the new mitochondrial donation technique.
Yet mitochondrial donation is distinct from surrogacy and cytoplasmic transfer for one simple reason: it is overtly intended to create children with DNA from three parents. Thus there is something inherently more unsettling about deliberately seeking to alter the inheritable DNA of a child. Unlike a course of drug treatment, genetic changes are permanent. The New York Times called the creation of “genetically modified babies” (an undeniably emotive descriptor) “a dangerous step” and an “extreme procedure” in a 2014 opinion piece by Marcy Darnovsky, Executive Director of the Center for Genetics and Society. Naturally, this led to fears that mitochondrial donation could lead to ‘designer babies’ (despite the fact that mitochondrial genes do not code for visible traits such as eye colour). A Republican representative for Nebraska, Jeff Fortenberry, went so far as to call it “a macabre form of eugenic cloning”.
Knee-jerk reactions aside, there are reasons to be cautious. Research is increasingly revealing that mitochondria are far more important than mere ‘batteries’, and have duties that include influencing the speed of nerve signalling, detoxifying ammonia in the liver, and playing a key role in programmed cell death.
Moreover, genetic information is continually shuttled between the nucleus and the mitochondria. This implies that shifting mitochondria from one woman to another could have unexpected consequences down the line.
The most distressing fact about mitochondrial replacement, however, may be that it will only work for a minority of families carrying mitochondrial diseases. We now know there are 1,000 – possibly 1,500 – genes in the DNA of a nucleus that code for proteins necessary for the creation of mitochondria. Yet many of these genes can also lead to faults. It is likely that only a quarter of all cases of mitochondrial disease can be attributable to genes within the mitochondria themselves.
“Even from very early on, more than 20 years ago, it was clear that most of the children with mitochondrial diseases don’t carry mitochondrial DNA mutations,” explains Rahman.
In other words, three-quarters of families carrying mitochondrial diseases somewhere in their lineage will not be able to use mitochondrial donation to protect their children. Nonetheless, the Human Fertilisation Embryology Authority carried out three scientific reviews of the treatment, and concluded that it was safe.
“It is a bold step for Parliament to take, but it is a considered and informed step,” MP Jane Ellison, Parliamentary Under-Secretary of State for Health, told the House of Commons in February 2015. “This is world-leading science within a highly respected regulatory regime, and for the families affected it is a light at the end of a very dark tunnel.”
Groundbreaking science
On 25 July 1978, Louise Brown, the first ever test tube baby, was born in Oldham General Hospital to her parents, John and Lesley. At the time, concerns were raised about ‘Frankenbabies’ and ‘playing God’, while certain members of the public subjected the Browns to hate mail and ridicule. Today, however, more than five million children have been born via IVF.
Ultimately, doctors are confident that this new technique will follow in the path of IVF to become a routine treatment that could transform lives.
Subscribe to BBC Focus magazine for fascinating new Q&As every month and follow @sciencefocusQA on Twitter for your daily dose of fun science facts.