While pharmaceutical giant Pfizer’s coronavirus vaccine has been found to be more than 90 per cent effective in preventing the disease, that does not mean life can return to normal just yet.
Here is everything you need to know about the new jab, what other coronavirus vaccines are in development and what the experts have to say.
Is the Pfizer coronavirus vaccine ready?
Yes!
The Pfizer vaccine has been approved for use in the UK by the Medicines and Healthcare products Regulatory Agency (MHRA).
Read more about coronavirus vaccines:
- Should we infect people with COVID-19 for vaccine research?
- COVID-19: Who should get a vaccine first?
Is the Pfizer coronavirus vaccine safe?
The coronavirus vaccine has been tested on 43,500 people in six countries and no safety concerns have been raised.
All vaccines undergo rigorous testing and have oversight from experienced regulators.
If trials go well, the next stage involves an expert review of all data by the MHRA or the European Medicines Agency (EMA).
Public safety comes first and regulators check that trials show a vaccine meets high standards of safety and effectiveness before granting a licence.
The MHRA said data to be reviewed must include results from the lab and clinical trials in humans; manufacturing and quality controls, product sampling, and testing of the final product. The factories where vaccines are made are also inspected before approval.
Once the MHRA has reviewed the data, it will seek advice from the Government’s independent advisory body, the Commission on Human Medicines.They will also critically assess the data before advising the Government on the safety, quality and effectiveness of any potential vaccine.

When will the UK get the Pfizer coronavirus vaccine?
The government announced on 2 December that “The vaccine will be made available across the UK from next week."
The UK has ordered 40 million doses of the Pfizer vaccine – the first agreement the firms signed with any government.
People will need two doses, meaning not enough shots have been secured for the entire UK population of over 66 million.
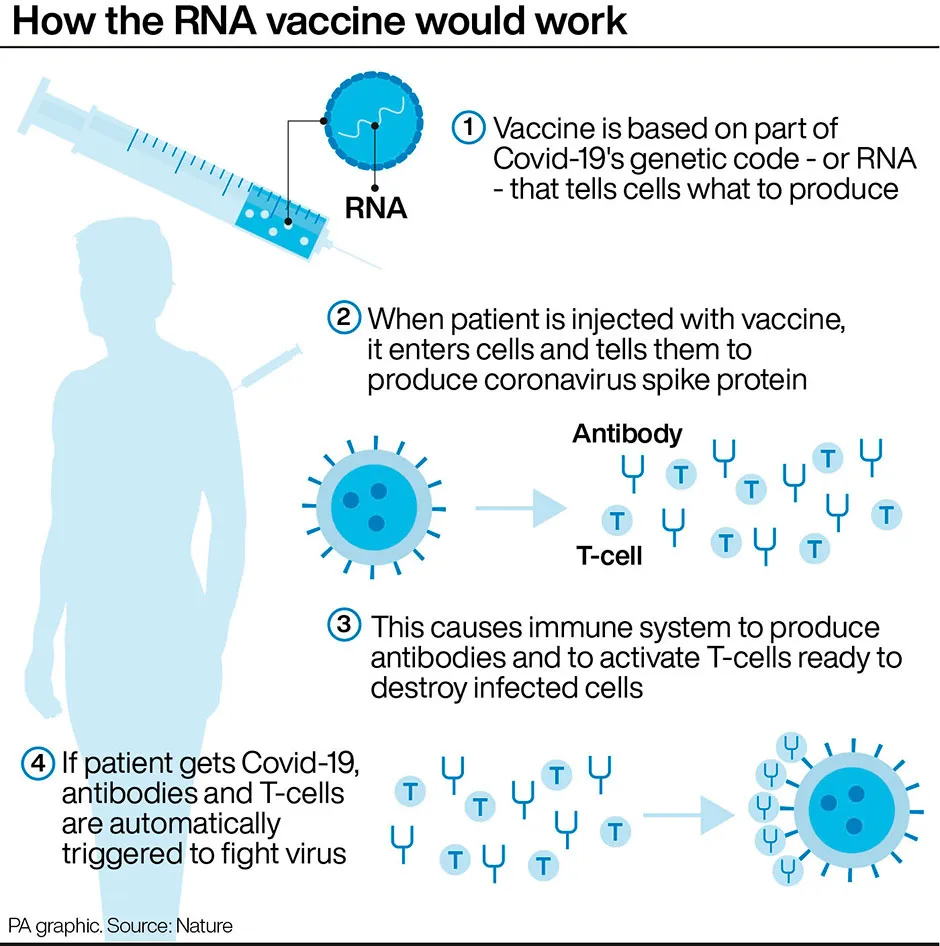
How does the Pfizer coronavirus vaccine work?
The Pfizer vaccine, made in conjunction with BioNTech, is known as a messenger RNA (mRNA) vaccine, which uses the virus’s genetic code rather than any part of the virus itself, and is injected into the body where it enters cells and tells them to create antigens.Conventional vaccines are produced using weakened forms of the virus.
Some believe mRNA vaccines are safer for the patient as they do not rely on any element of the virus being injected into the body.
Are there problems with the Pfizer coronavirus vaccine?
The Pfizer COVID vaccine could pose some “logistical issues” if it is approved for widespread use because it needs to be stored at ultra low temperatures, experts have said.
Many vaccines which require cold storage need to be kept at around 4°C or lower but it has been suggested that the Pfizer vaccine needs to be stored at -70°C. This could pose problems for transport and storage of the vaccine.
“With all vaccines, ensuring that they are stored distributed and administered properly is essential, and huge efforts are under way to ensure this is possible for new vaccines," said Dr Alexander Edwards, associate professor in biomedical technology at the University of Reading.
“For example, the task of producing substantial amounts of a new vaccine and distributing widely will be a challenge, not least for this particular formulation where ensuring that it can be appropriately frozen until needed and must not be allowed to thaw in transit."
Read the latest coronavirus news:
- COVID-19: How many people have died because of the pandemic?
- COVID-19 antibodies halve within three months, latest Oxford study suggests
- 'Persistent' structural changes in lungs could explain long COVID
But the NHS has said it has been preparing for a vaccine delivery.Last week NHS England chief executive Simon Stevens acknowledged that some vaccine candidates need ultra low storage.
“So it’s going to be a combination of what GPs are able to do, what community pharmacists are able to do, but also mass vaccination centres, which is one of the purposes we will be using the Nightingale Hospitals for, and other locations as well," he told a press conference.
“There will be roving teams who will prioritise care homes and social care staff and other vulnerable groups.”
Data released about the Pfizer vaccine does not indicate how long immunity lasts, but suggests protection is achieved 28 days after vaccination.
It is also not yet known how well the vaccine works in elderly people, who are at the highest risk.
What other coronavirus vaccines are there?
There are currently more than 200 coronavirus vaccine candidates being tested around the world.
About 12 COVID-19 vaccines around the world are currently in the final stages of testing. There are two frontrunners in the race – the one from Pfizer, called BNT162b2, and another being developed by the University of Oxford and AstraZeneca, which is also in phase three clinical trials.
Over 20,000 volunteers are now participating in trials for the Oxford vaccine, which are taking place in countries including the UK, South Africa, Brazil and Kenya. The vaccine, called ChAdOx1 nCoV-19, uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
Professor Andrew Pollard, head of Oxford’s vaccine trial team, said he is optimistic data on safety and efficacy of their vaccine will be available by the end of the year.
Other potential vaccines in phase three trials include ones by US drugs firm Moderna and biotech company Novavax.
