Early data for Russia’s coronavirus vaccinesuggests it is 92 per cent effective, the country’s sovereign wealth fund has said. These results have not been peer-reviewed.
The announcement on the Sputnik V vaccine comes days after pharmaceutical giant Pfizer and biotech firm BioNTech released interim results (also not yet peer-reviewed) suggesting their vaccine is more than 90 per cent effective at preventing COVID-19.
The Phase 3 trials evaluated efficacy among more than 16,000 volunteers who received the vaccine or placebo 21 days after the first injection.
Statistical analysis of 20 confirmed cases of coronavirus, the cases split between vaccinated individuals and those who received the placebo, indicates the vaccine had an efficacy rate of 92 per cent after the second dose.
The Russian Direct Investment Fund (RDIF), which has been backing the vaccine, said there were no unexpected adverse events during the trials, and monitoring of the participants is continuing.
Read more about the vaccines in development:
- Everything you need to know about the Pfizer coronavirus vaccine
- Oxford vaccine could be put before regulators by the end of the year
- Coronavirus: Imperial vaccine could be approved by mid-2021
The Phase 3 study of the vaccine, developed by the Gamaleya National Center, is taking place in 29 clinics across Moscow and will involve 40,000 volunteers in total, with a quarter receiving a placebo shot.
“Positive interim results of Phase 3 give reasons to expect a successful outcome of Sputnik V clinical trials,” said Denis Logunov, deputy director of the Gamaleya Center.
“We will continue to process and analyse all the data and look to the future with optimism, expecting that results of our work will help end the pandemic sooner.”
However, some scientists have raised suspicions at the timing of the announcement.
“I worry that these data have been rushed out on the back of the Pfizer/BioNtech announcement earlier in the week,” said Eleanor Riley, a professor of immunology and infectious disease at the University of Edinburgh. “The Sputnik data are based on only 20 cases of COVID-19 in the trial participants, compared to more than 90 cases in the earlier trial.
“This is not a competition. We need all trials to be a carried out to the highest possible standards and it is particularly important that the pre-set criteria for unblinding the trial data are adhered to avoid cherry-picking the data. Anything less than this risks a public loss of trust in all vaccines, which would be a disaster.”
How does the Russian coronavirus vaccine work?
“This vaccine uses an inactivated adenoviral vector (a carrier) – a well established method used in gene therapy and cancer research for decades,” said Dr Gillies O’Bryan-Tear, of the Faculty of Pharmaceutical Medicine.
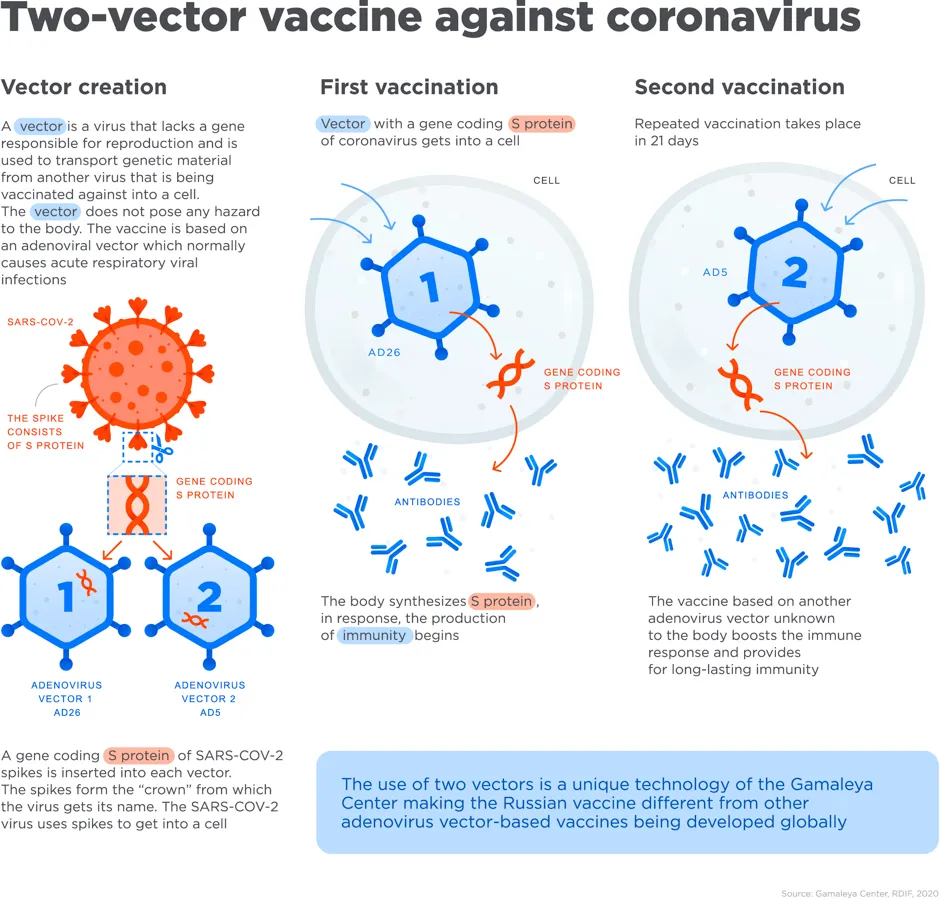
“The viral vector is used to carry DNA sequences coding for the spike protein of the COVID-19 virus into the body’s cells, which then generate the spike protein antigen using the cell’s own mRNA. This antigen is expressed on the cell surface where it can be recognised by the body’s immune system.
“This is a different type of vaccine to the Pfizer/BioNtech vaccine, although it targets the same part of the virus. The inactviated viral vector cannot replicate in the human body so poses little health risk.”
What is the difference between the Russian Sputnik V and the Pfizer vaccine?
The type, administration and stability of the vaccines differ.
“Whereas the Pfizer/BioNTech vaccine is based on RNA that produces a fragment of the SARS-CoV-2 S (spike) protein, the Sputnik V vaccine consists of two doses, three weeks apart, respectively of two different human adenoviruses, each one expressing the S protein,” said Prof Charles Bangham, chair of immunology at Imperial College London.
“The Sputnik V strategy has some theoretical advantages, because the use of the full-length S protein may elicit a broader immune response, but the adenoviruses used are also likely to produce more side-effects such as fever or headache – although these are expected to be mild. However, proper evaluation of the safety and efficacy of each of these two vaccines, the duration of protection, and their effectiveness in the elderly, must await publication of the full data on the trials.”

Previous results have suggested that the Russian vaccine approach does not need to be kept at the same temperatures as the Pfizer vaccine, which needs to be frozen at around-70°C to be transported.
What is next for the Russian vaccine?
“The Sputnik data is yet more good news for COVID-19 vaccine development,” said Ian Jones, professor of virology at the University of Reading.
“Although based on fewer cases than the recent Pfizer data, the vaccine looks as efficient and, like the Pfizer data, confirms and extends the earlier phase two results.
“We still need to know about the longevity of the response and the efficiency in different age groups, but the result bodes well for the other trials currently in progress and for having enough vaccine in geographically diverse regions to enable a comprehensive vaccination programme on a global scale.”
Read the latest coronavirus news:
